Live functional assays reveal longitudinal maturation of transepithelial transport in kidney organoids
- PMID: 36046340
- PMCID: PMC9420851
- DOI: 10.3389/fcell.2022.978888
Live functional assays reveal longitudinal maturation of transepithelial transport in kidney organoids
Abstract
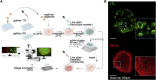
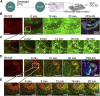
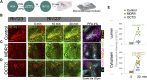
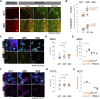
Kidney organoids derived from hPSCs have opened new opportunities to develop kidney models for preclinical studies and immunocompatible kidney tissues for regeneration. Organoids resemble native nephrons that consist of filtration units and tubules, yet little is known about the functional capacity of these organoid structures. Transcriptomic analyses provide insight into maturation and transporter activities that represent kidney functions. However, functional assays in organoids are necessary to demonstrate the activity of these transport proteins in live tissues. The three-dimensional (3D) architecture adds complexity to real-time assays in kidney organoids. Here, we develop a functional assay using live imaging to assess transepithelial transport of rhodamine 123 (Rh123), a fluorescent substrate of P-glycoprotein (P-gp), in organoids affixed to coverslip culture plates for accurate real-time observation. The identity of organoid structures was probed using Lotus Tetragonolobus Lectin (LTL), which binds to glycoproteins present on the surface of proximal tubules. Within 20 min of the addition of Rh123 to culture media, Rh123 accumulated in the tubular lumen of organoids. Basolateral-to-apical accumulation of the dye/marker was reduced by pharmacologic inhibition of MDR1 or OCT2, and OCT2 inhibition reduced the Rh123 uptake. The magnitude of Rh123 transport was maturation-dependent, consistent with MDR1 expression levels assessed by RNA-seq and immunohistochemistry. Specifically, organoids on day 21 exhibit less accumulation of Rh123 in the lumen unlike later-stage organoids from day 30 of differentiation. Our work establishes a live functional assessment in 3D kidney organoids, enabling the functional phenotyping of organoids in health and disease.
Keywords: functional assay; kidney; nephron; organoid; real-time imaging; tubular transport.
Copyright © 2022 Rizki-Safitri, Gupta, Hiratsuka, Kobayashi, Zhang, Ida, Satlin and Morizane.
Conflict of interest statement
RM is a co-inventor on patents (PCT/US2018/036677) that are assigned to President and Fellows of Harvard College and Mass General Brigham. RM serves on the Scientific Advisory Board of Trestle Biotherapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

