Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: central composite design optimization, in- silico and in-vivo studies
- PMID: 36047012
- PMCID: PMC9448385
- DOI: 10.1080/10717544.2022.2108939
Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: central composite design optimization, in- silico and in-vivo studies
Abstract
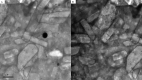
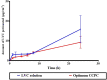
Levocetirizine hydrochloride (LVC) is an antihistaminic drug that is repurposed for the treatment of alopecia. This investigation is targeted for formulating LVC into cationic ceramide/phospholipid composite (CCPCs) for the management of alopecia. CCPCs were fabricated by ethanol-injection approach, through a central composite experiment. CCPCs were evaluated by inspecting their entrapment efficiency (EE%), polydispersity index (PDI), particle size (PS), and zeta potential (ZP). The optimum CCPCs were additionally studied by in-vitro, ex-vivo, in-silico, and in-vivo studies. The fabricated CCPCs had acceptable EE%, PS, PDI, and ZP values. The statistical optimization elected optimum CCPCs composed of 5 mg hyaluronic acid, 10 mg ceramide III, and 5 mg dimethyldidodecylammonium bromide employing phytantriol as a permeation enhancer. The optimum CCPCs had EE%, PS, PDI, and ZP of 88.36 ± 0.34%, 479.00 ± 50.34 nm, 0.377 ± 0.0035, and 20.20 ± 1.13 mV, respectively. The optimum CCPC maintained its stability for up to 90 days. It also viewed vesicles of tube shape via transmission electron microscope. The in-silico assessment resulted in better interaction and stability between LVC and vesicle components in water. The ex-vivo and in-vivo assessments showed satisfactory skin retention of LVC from optimum CCPCs. The histopathological assessment verified the safety of optimum CCPCs to be topically applied. Overall, the optimum CCPCs could be utilized as a potential system for the topical management of alopecia, with a prolonged period of activity, coupled with reduced LVC shortcomings.
Keywords: Alopecia; ceramide; drug discovery; in-silico study; industrial development; levocetirizine hydrochloride.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


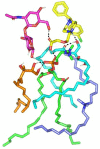




References
-
- Abd-Elsalam WH, El-Helaly SN, Ahmed MA, Al-mahallawi AM. (2018). Preparation of novel phospholipid-based sonocomplexes for improved intestinal permeability of rosuvastatin: in vitro characterization, dynamic simulation, Caco-2 cell line permeation and in vivo assessment studies. Int. J. Pharm 548:375–84. - PubMed
-
- Abdelgawad R, Nasr M, Moftah NH, Hamza MY. (2017). Phospholipid membrane tubulation using ceramide doping “Cerosomes”: characterization and clinical application in psoriasis treatment. Eur J Pharm Sci 101:258–68. - PubMed
-
- Abdellatif MM, Khalil IA, Khalil MAF. (2017). Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: in-vitro, ex-vivo and in-vivo evaluation. Int J Pharm 527:1–11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
