Clinical Performance of the RealTi m e High Risk HPV Assay on Self-Collected Vaginal Samples within the VALHUDES Framework
- PMID: 36047900
- PMCID: PMC9602690
- DOI: 10.1128/spectrum.01631-22
Clinical Performance of the RealTi m e High Risk HPV Assay on Self-Collected Vaginal Samples within the VALHUDES Framework
Abstract
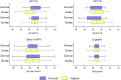
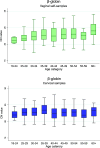
The VALHUDES framework (NCT03064087) was established to evaluate the clinical accuracy of HPV testing on self-samples compared with HPV testing on matched clinician-taken cervical samples. Women referred to colposcopy due to previous cervical abnormalities were recruited at five Belgian colposcopy centers. A total of 486 pairs of matched cervical samples and vaginal self-samples were included in the analysis (228 collected with Evalyn Brush and 258 with Qvintip). The dry vaginal brushes were transferred into 20 mL ThinPrep PreservCyt solution. All specimens were tested with the Abbott RealTime High Risk HPV assay (Abbott RT). Testing on vaginal and cervical specimens was considered the index and comparator tests, respectively, and colposcopy and histology as the reference standard. The clinical sensitivity for CIN2+ of Abbott RT (cutoff ≤32 cycle number [CN]) on vaginal self-samples (Evalyn Brush and Qvintip combined) was 8% lower than on the cervical clinician-collected samples (ratio = 0.92 [95% CI, 0.87 to 0.98]), while the specificity was similar (ratio = 1.04 [95% CI, 0.97 to 1.12]). Sensitivity (ratio = 0.95 [95% CI, 0.89 to 1.02]) and specificity (ratio = 1.11 [95% CI, 0.995 to 1.23]) on Evalyn Brush samples was similar to cervical, while on Qvintip samples, the sensitivity was 12% lower than cervical samples (ratio = 0.88 [95% CI, 0.78 to 0.998]) with similar specificity (0.99 [95% CI, 0.90 to 1.10]). Exploratory cutoff optimization (cutoff ≤35 CN) resulted in an improvement of the relative sensitivity (self-sampling versus clinician sampling: ratio = 0.96 [95% CI, 0.91 to 1.02]) but yielded a loss in relative specificity (ratio = 0.92 [0.85 to 1.00]). The clinical accuracy of Abbott RT differed from the self-sampling device. However, after cutoff optimization, the sensitivity on self-samples taken with either of two vaginal brushes became similar to clinician-collected samples. IMPORTANCE Self-samples are becoming a crucial part of HPV-based cervical cancer screening programs to reach nonattendee women and increase screening coverage. Therefore, the VALHUDES framework was established to validate and evaluate HPV tests and devices on self-samples. Here, in the present manuscript, we evaluated the accuracy of the RealTime High Risk HPV assay (Abbott RT) on two different vaginal devices to detect cervical intraepithelial neoplasia grade two or higher (CIN2+). The study results demonstrated that the Abbott RT assay is similarly accurate on vaginal self-samples as on matched clinician-taken cervical samples after adjusting cutoff values. Moreover, we observed that some vaginal devices perform better than others in CIN2+ detection. We also underline the necessity of standardization and validation of general workflow and sample handling procedures for vaginal self-samples.
Keywords: Abbott RealTime High Risk HPV assay; HPV; VALHUDES; cervical cancer screening; diagnostic test accuracy; self-sampling.
Conflict of interest statement
The authors declare a conflict of interest. The VALHUDES project is a researcher-induced study, designed by Sciensano (Principal Investigator; Brussels, Belgium), CEV (University of Antwerp, Antwerp, Belgium), and AML (Antwerp, Belgium). HPV assays and devices manufacturers can participate in the VALHUDES framework contributing financial support and equipment for laboratory testing and statistical analysis. This research was supported by a grant from Abbott Laboratories (Abbott GmbH, Wiesbaden, Germany), Novosanis NV (Wijnegem, Belgium), University of Antwerp (Antwerp, Belgium). The study group received sample collection devices from Rovers Medical Devices B.V. (Oss, The Netherlands) and Aprovix AB (Uppsala, Sweden).
Figures
Similar articles
-
Validation of BD Onclarity HPV Assay on Vaginal Self-Samples versus Cervical Samples Using the VALHUDES Protocol.Cancer Epidemiol Biomarkers Prev. 2022 Dec 5;31(12):2177-2184. doi: 10.1158/1055-9965.EPI-22-0757. Cancer Epidemiol Biomarkers Prev. 2022. PMID: 36099441
-
Direct comparison of two vaginal self-sampling devices for the detection of human papillomavirus infections.J Clin Virol. 2016 Sep;82:46-50. doi: 10.1016/j.jcv.2016.06.016. Epub 2016 Jun 28. J Clin Virol. 2016. PMID: 27434147
-
Comparison of the Clinical Accuracy of Xpert HPV Assay on Vaginal Self-Samples and Cervical Clinician-Taken Samples within the VALHUDES Framework.J Mol Diagn. 2023 Sep;25(9):702-708. doi: 10.1016/j.jmoldx.2023.06.004. Epub 2023 Jun 22. J Mol Diagn. 2023. PMID: 37354994
-
Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis.Lancet Oncol. 2014 Feb;15(2):172-83. doi: 10.1016/S1470-2045(13)70570-9. Epub 2014 Jan 14. Lancet Oncol. 2014. PMID: 24433684 Review.
-
Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: a systematic review and meta-analysis.Lancet Oncol. 2022 Jul;23(7):950-960. doi: 10.1016/S1470-2045(22)00294-7. Epub 2022 Jun 13. Lancet Oncol. 2022. PMID: 35709810
Cited by
-
Accuracy of Liferiver HarmoniaHPV and VenusHPV Assays on Urine and Vaginal Self-Samples.J Med Virol. 2025 Mar;97(3):e70273. doi: 10.1002/jmv.70273. J Med Virol. 2025. PMID: 40028694 Free PMC article.
-
Comparison of diagnostic accuracy and acceptability of self-sampling devices for human Papillomavirus detection: A systematic review.Prev Med Rep. 2024 Jan 4;38:102590. doi: 10.1016/j.pmedr.2024.102590. eCollection 2024 Feb. Prev Med Rep. 2024. PMID: 38283967 Free PMC article. Review.
-
Variables that impact HPV test accuracy during vaginal self collection workflow for cervical cancer screening.Gynecol Oncol Rep. 2024 May 25;54:101421. doi: 10.1016/j.gore.2024.101421. eCollection 2024 Aug. Gynecol Oncol Rep. 2024. PMID: 38881560 Free PMC article. Review.
-
Revolutionizing Cervical Cancer Screening: Self-Vaginal Sampling for Human Papillomavirus Detection.South Asian J Cancer. 2024 Aug 22;14(1):67-68. doi: 10.1055/s-0044-1789274. eCollection 2025 Jan. South Asian J Cancer. 2024. PMID: 40124159 Free PMC article.
References
-
- Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, Ivanuš U, McKee M, de Koning HJ, de Kok IMCM, EU-TOPIA consortium . 2020. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer 127:207–223. doi:10.1016/j.ejca.2019.12.013. - DOI - PubMed
-
- Landy R, Sasieni PD, Mathews C, Wiggins CL, Robertson M, McDonald YJ, Goldberg DW, Scarinci IC, Cuzick J, Wheeler CM, New Mexico HPV Pap Registry Steering Committee . 2020. Impact of screening on cervical cancer incidence: a population-based case-control study in the United States. Int J Cancer 147:887–896. doi:10.1002/ijc.32826. - DOI - PMC - PubMed

