Ocrelizumab effect on humoral and cellular immunity in multiple sclerosis and its clinical correlates: a 3-year observational study
- PMID: 36048265
- PMCID: PMC9813008
- DOI: 10.1007/s00415-022-11350-1
Ocrelizumab effect on humoral and cellular immunity in multiple sclerosis and its clinical correlates: a 3-year observational study
Abstract
Objective: We aim to evaluate 3-year effects of ocrelizumab (humanized anti-CD20 monoclonal antibody for the treatment of multiple sclerosis (MS)) on lymphocytes, neutrophils and immunoglobulins: (1) when compared with pre-infusion assessment; (2) over the course of treatment; and (3) possible clinical correlates of the observed immunological modifications.
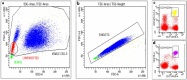
Methods: This real-world observational cohort study has been conducted on prospectively collected data from 78 MS patients (mean age 47.8 ± 10.5 years; females 48.7%) commencing on ocrelizumab from 2018, with mean follow-up of 36.5 ± 6.8 months. Clinical data and blood samples were collected every three months. Total lymphocyte count and subpopulations were assessed on peripheral blood using flow cytometry. Serum immunoglobulins were evaluated with nephelometry.
Results: When compared with pre-infusion values, we observed reduction of total, CD19 and CD20 lymphocyte counts; however, after the first infusion, their levels remained substantially stable. Over time we observed a progressive reduction of CD8 lymphocytes, while no changes were observed for CD4, CD27, CD3CD27, and CD19CD27. After the first infusion, we observed reduction in IgG, which further decreased during the follow-up. Higher probability of EDSS progression was associated with reduced modulation of CD8 lymphocytes.
Interpretation: Ocrelizumab affects both humoral and cellular immune responses. Disability progression over the follow-up was associated with lower CD8 cytotoxic T-lymphocyte reduction. Changes in humoral response are immediate and sustained, while modulation of cellular immunity occurs progressively through regular re-treatment, and is related to clinical stability.
Keywords: Immunoglobulins; Lymphocytes; Multiple sclerosis; Ocrelizumab.
© 2022. The Author(s).
Conflict of interest statement
RL has received honoraria from Biogen, Merck, Novartis, Roche, and Teva. MP discloses travel/meeting expenses from Novartis, Roche and Merck, speaking honoraria from HEALTH&LIFE S.r.l. and honoraria for consulting services from Biogen. VBM has received research grants from the Italian MS Society, and Roche, and honoraria from Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. MM has received research grants from the ECTRIMS-MAGNIMS, the UK MS Society, and Merck, and honoraria from Biogen, Merck, Novartis and Roche. Other authors have nothing to disclose.
Figures
Similar articles
-
Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis.Mult Scler Relat Disord. 2021 Apr;49:102802. doi: 10.1016/j.msard.2021.102802. Epub 2021 Jan 28. Mult Scler Relat Disord. 2021. PMID: 33556652
-
Real-world effectiveness, safety and immunogenicity of ocrelizumab in turkish multiple sclerosis patients: a single-center experience for 4-year follow-up.Acta Neurol Belg. 2024 Aug;124(4):1385-1391. doi: 10.1007/s13760-024-02572-3. Epub 2024 May 20. Acta Neurol Belg. 2024. PMID: 38769274 Review.
-
Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience.Int J Mol Sci. 2024 May 14;25(10):5353. doi: 10.3390/ijms25105353. Int J Mol Sci. 2024. PMID: 38791391 Free PMC article.
-
Humoral and T-cell responses to SARS-CoV-2 vaccination in multiple sclerosis patients treated with ocrelizumab.Mult Scler Relat Disord. 2022 Jan;57:103382. doi: 10.1016/j.msard.2021.103382. Epub 2021 Nov 9. Mult Scler Relat Disord. 2022. PMID: 35158475 Free PMC article.
-
Immunomodulatory effects of ocrelizumab and candidate biomarkers for monitoring treatment response in multiple sclerosis.Mult Scler. 2023 Jun;29(7):779-788. doi: 10.1177/13524585221147635. Epub 2023 Jan 22. Mult Scler. 2023. PMID: 36683286 Review.
Cited by
-
The ocrelizumab wearing-off phenomenon is associated with reduced immunomodulatory response and increased neuroaxonal damage in multiple sclerosis.J Neurol. 2024 Aug;271(8):5012-5024. doi: 10.1007/s00415-024-12434-w. Epub 2024 May 23. J Neurol. 2024. PMID: 38777960 Free PMC article.
-
Optimizing premedications for multiple sclerosis patients treated with ocrelizumab: A randomized controlled trial.Mult Scler J Exp Transl Clin. 2025 Aug 10;11(3):20552173251359074. doi: 10.1177/20552173251359074. eCollection 2025 Jul-Sep. Mult Scler J Exp Transl Clin. 2025. PMID: 40799203 Free PMC article.
-
Dynamic Evolution of Humoral and T-Cell Specific Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Followed until the Booster Dose.Int J Mol Sci. 2023 May 10;24(10):8525. doi: 10.3390/ijms24108525. Int J Mol Sci. 2023. PMID: 37239872 Free PMC article.
-
Long-term use of rituximab increases T cell count in MS patients.Front Immunol. 2024 Jul 17;15:1412668. doi: 10.3389/fimmu.2024.1412668. eCollection 2024. Front Immunol. 2024. PMID: 39086478 Free PMC article.
-
Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with anti-CD20 treatments: a systematic review and meta-analysis of 19,139 multiple sclerosis patients.Front Neurol. 2024 Apr 18;15:1380654. doi: 10.3389/fneur.2024.1380654. eCollection 2024. Front Neurol. 2024. PMID: 38699050 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

