Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial
- PMID: 36048453
- PMCID: PMC9437831
- DOI: 10.1001/jamaoncol.2022.3829
Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial
Erratum in
-
Errors in Figure 1.JAMA Oncol. 2023 Jan 1;9(1):151. doi: 10.1001/jamaoncol.2022.6629. JAMA Oncol. 2023. PMID: 36416803 Free PMC article. No abstract available.
Abstract
Importance: Early results at 3 years from the PRODIGE 24/Canadian Cancer Trials Group PA6 randomized clinical trial showed survival benefits with adjuvant treatment with modified FOLFIRINOX vs gemcitabine in patients with resected pancreatic ductal adenocarcinoma; mature data are now available.
Objective: To report 5-year outcomes and explore prognostic factors for overall survival.
Design, setting, and participants: This open-label, phase 3 randomized clinical trial was conducted at 77 hospitals in France and Canada and included patients aged 18 to 79 years with histologically confirmed pancreatic ductal adenocarcinoma who had undergone complete macroscopic (R0/R1) resection within 3 to 12 weeks before randomization. Patients were included from April 16, 2012, through October 3, 2016. The cutoff date for this analysis was June 28, 2021.
Interventions: A total of 493 patients were randomized (1:1) to receive treatment with modified FOLFIRINOX (oxaliplatin, 85 mg/m2 of body surface area; irinotecan, 150-180 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 2400 mg/m2, every 2 weeks) or gemcitabine (1000 mg/m2, days 1, 8, and 15, every 4 weeks) as adjuvant therapy for 24 weeks.
Main outcomes and measures: Primary end point was disease-free survival. Secondary end points included overall survival, metastasis-free survival, and cancer-specific survival. Prognostic factors for overall survival were determined.
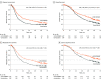
Results: Of the 493 patients, 216 (43.8%) were women, and the mean (SD) age was 62.0 (8.9) years. At a median of 69.7 months' follow-up, 367 disease-free survival events were observed. In patients receiving chemotherapy with modified FOLFIRINOX vs gemcitabine, median disease-free survival was 21.4 months (95% CI, 17.5-26.7) vs 12.8 months (95% CI, 11.6-15.2) (hazard ratio [HR], 0.66; 95% CI, 0.54-0.82; P < .001) and 5-year disease-free survival was 26.1% vs 19.0%; median overall survival was 53.5 months (95% CI, 43.5-58.4) vs 35.5 months (95% CI, 30.1-40.3) (HR, 0.68; 95% CI, 0.54-0.85; P = .001), and 5-year overall survival was 43.2% vs 31.4%; median metastasis-free survival was 29.4 months (95% CI, 21.4-40.1) vs 17.7 months (95% CI, 14.0-21.2) (HR, 0.64; 95% CI, 0.52-0.80; P < .001); and median cancer-specific survival was 54.7 months (95% CI, 45.8-68.4) vs 36.3 months (95% CI, 30.5-43.9) (HR, 0.65; 95% CI, 0.51-0.82; P < .001). Multivariable analysis identified modified FOLFIRINOX, age, tumor grade, tumor staging, and larger-volume center as significant favorable prognostic factors for overall survival. Shorter relapse delay was an adverse prognostic factor.
Conclusions and relevance: The final 5-year results from the PRODIGE 24/Canadian Cancer Trials Group PA6 randomized clinical trial indicate that adjuvant treatment with modified FOLFIRINOX yields significantly longer survival than gemcitabine in patients with resected pancreatic ductal adenocarcinoma.
Trial registration: EudraCT: 2011-002026-52; ClinicalTrials.gov Identifier: NCT01526135.
Conflict of interest statement
Figures

References
-
- Globocan 2020 . Pancreas fact sheet. Accessed February 21, 2022. https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

