Specific binding of Hsp27 and phosphorylated Tau mitigates abnormal Tau aggregation-induced pathology
- PMID: 36048712
- PMCID: PMC9436411
- DOI: 10.7554/eLife.79898
Specific binding of Hsp27 and phosphorylated Tau mitigates abnormal Tau aggregation-induced pathology
Abstract
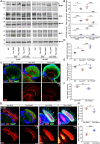
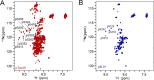
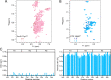
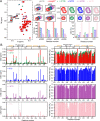
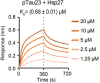
Amyloid aggregation of phosphorylated Tau (pTau) into neurofibrillary tangles is closely associated with Alzheimer's disease (AD). Several molecular chaperones have been reported to bind Tau and impede its pathological aggregation. Recent findings of elevated levels of Hsp27 in the brains of patients with AD suggested its important role in pTau pathology. However, the molecular mechanism of Hsp27 in pTau aggregation remains poorly understood. Here, we show that Hsp27 partially co-localizes with pTau tangles in the brains of patients with AD. Notably, phosphorylation of Tau by microtubule affinity regulating kinase 2 (MARK2), dramatically enhances the binding affinity of Hsp27 to Tau. Moreover, Hsp27 efficiently prevents pTau fibrillation in vitro and mitigates neuropathology of pTau aggregation in a Drosophila tauopathy model. Further mechanistic study reveals that Hsp27 employs its N-terminal domain to directly interact with multiple phosphorylation sites of pTau for specific binding. Our work provides the structural basis for the specific recognition of Hsp27 to pathogenic pTau, and highlights the important role of Hsp27 in preventing abnormal aggregation and pathology of pTau in AD.
Keywords: Alzheimer's disease; E. coli; Hsp27; biochemistry; chaperone; chemical biology; human; phosphorylated Tau; tauopathy.
© 2022, Zhang, Zhu, Lu et al.
Conflict of interest statement
SZ, YZ, JL, ZL, AL, WZ, JL, JQ, SZ, YZ, CL, JL, ZH, RZ, DL No competing interests declared
Figures






References
-
- Abisambra JF, Blair LJ, Hill SE, Jones JR, Kraft C, Rogers J, Koren J, Jinwal UK, Lawson L, Johnson AG, Wilcock D, O’Leary JC, Jansen-West K, Muschol M, Golde TE, Weeber EJ, Banko J, Dickey CA. Phosphorylation dynamics regulate hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. The Journal of Neuroscience. 2010;30:15374–15382. doi: 10.1523/JNEUROSCI.3155-10.2010. - DOI - PMC - PubMed
-
- Ando K, Maruko-Otake A, Ohtake Y, Hayashishita M, Sekiya M, Iijima KM. Stabilization of microtubule-unbound tau via tau phosphorylation at ser262/356 by par-1/MARK contributes to augmentation of AD-related phosphorylation and aβ42-induced tau toxicity. PLOS Genetics. 2016;12:e1005917. doi: 10.1371/journal.pgen.1005917. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

