National Vaccination Coverage Among Adolescents Aged 13-17 Years - National Immunization Survey-Teen, United States, 2021
- PMID: 36048724
- PMCID: PMC9472778
- DOI: 10.15585/mmwr.mm7135a1
National Vaccination Coverage Among Adolescents Aged 13-17 Years - National Immunization Survey-Teen, United States, 2021
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
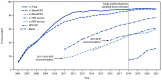
Figures

References
-
- CDC. COVID-19 vaccines for children and teens. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed August 29, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/child...
-
- Williams CL, Walker TY, Elam-Evans LD, et al. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey-Teen, 2016-2017. Hum Vaccin Immunother 2020;16:562–72. 10.1080/21645515.2019.1670036 - DOI - PMC - PubMed
-
- Rural Health Information Hub. Effective communication and consistency in increasing rural vaccination rates. Grand Forks, ND: The Rural Monitor; 2019. https://www.ruralhealthinfo.org/rural-monitor/increasing-vaccination-rates/
-
- Sparks G, Hamel L, Kirzinger A, Stokes M, Brodie M. KFF COVID-19 vaccine monitor: differences in vaccine attitudes between rural, suburban, and urban areas. San Francisco, CA: KFF; 2021. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vacci...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

