SARS-CoV-2 requires acidic pH to infect cells
- PMID: 36048924
- PMCID: PMC9499588
- DOI: 10.1073/pnas.2209514119
SARS-CoV-2 requires acidic pH to infect cells
Expression of concern in
-
Editorial Expression of Concern: SARS-CoV-2 requires acidic pH to infect cells.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2417740121. doi: 10.1073/pnas.2417740121. Epub 2024 Oct 7. Proc Natl Acad Sci U S A. 2024. PMID: 39374403 Free PMC article. No abstract available.
Abstract
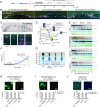
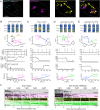
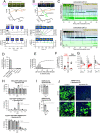
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry starts with membrane attachment and ends with spike (S) protein-catalyzed membrane fusion depending on two cleavage steps, namely, one usually by furin in producing cells and the second by TMPRSS2 on target cells. Endosomal cathepsins can carry out both. Using real-time three-dimensional single-virion tracking, we show that fusion and genome penetration require virion exposure to an acidic milieu of pH 6.2 to 6.8, even when furin and TMPRSS2 cleavages have occurred. We detect the sequential steps of S1-fragment dissociation, fusion, and content release from the cell surface in TMPRRS2-overexpressing cells only when exposed to acidic pH. We define a key role of an acidic environment for successful infection, found in endosomal compartments and at the surface of TMPRSS2-expressing cells in the acidic milieu of the nasal cavity.
Keywords: 3D imaging; SARS-CoV-2; infection route; live-cell imaging; virus entry.
Conflict of interest statement
Competing interest statement: T.K. is a member of the Medical Advisory Board of AI Therapeutics, Inc.
Figures


Update of
-
SARS-CoV-2 requires acidic pH to infect cells.bioRxiv [Preprint]. 2022 Jun 14:2022.06.09.495472. doi: 10.1101/2022.06.09.495472. bioRxiv. 2022. Update in: Proc Natl Acad Sci U S A. 2022 Sep 20;119(38):e2209514119. doi: 10.1073/pnas.2209514119. PMID: 35702155 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

