GnRH replacement rescues cognition in Down syndrome
- PMID: 36048943
- PMCID: PMC7613827
- DOI: 10.1126/science.abq4515
GnRH replacement rescues cognition in Down syndrome
Abstract
At the present time, no viable treatment exists for cognitive and olfactory deficits in Down syndrome (DS). We show in a DS model (Ts65Dn mice) that these progressive nonreproductive neurological symptoms closely parallel a postpubertal decrease in hypothalamic as well as extrahypothalamic expression of a master molecule that controls reproduction-gonadotropin-releasing hormone (GnRH)-and appear related to an imbalance in a microRNA-gene network known to regulate GnRH neuron maturation together with altered hippocampal synaptic transmission. Epigenetic, cellular, chemogenetic, and pharmacological interventions that restore physiological GnRH levels abolish olfactory and cognitive defects in Ts65Dn mice, whereas pulsatile GnRH therapy improves cognition and brain connectivity in adult DS patients. GnRH thus plays a crucial role in olfaction and cognition, and pulsatile GnRH therapy holds promise to improve cognitive deficits in DS.
Conflict of interest statement
Figures

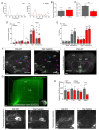

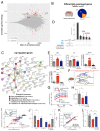



Comment in
-
Boosting cognition with a hormone.Science. 2022 Sep 2;377(6610):1042-1043. doi: 10.1126/science.add9456. Epub 2022 Sep 1. Science. 2022. PMID: 36048939
-
Gonadotropin releasing hormone (GnRH): a hormone therapy boosts cognition in Down syndrome and dementia.Signal Transduct Target Ther. 2023 Feb 1;8(1):49. doi: 10.1038/s41392-023-01321-x. Signal Transduct Target Ther. 2023. PMID: 36725840 Free PMC article. No abstract available.
References
-
- de la Torre R, et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:801–810. - PubMed
-
- Shapiro BL. Down syndrome--a disruption of homeostasis. Am J Med Genet. 1983;14:241–269. - PubMed
-
- Bull MJ. Down Syndrome. N Engl J Med. 2020;382:2344–2352. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

