Validating the Simplified Endoscopic Mucosal Assessment for Crohn's Disease: A Novel Method for Assessing Disease Activity
- PMID: 36049024
- PMCID: PMC10320367
- DOI: 10.1093/ibd/izac183
Validating the Simplified Endoscopic Mucosal Assessment for Crohn's Disease: A Novel Method for Assessing Disease Activity
Abstract
Background: To demonstrate treatment efficacy in Crohn's disease (CD), regulatory authorities require that trials include an endoscopic remission/response end point; however, standardized endoscopic assessment of disease activity, such as the Simple Endoscopic Score for Crohn's Disease (SES-CD), is not typically recorded by clinicians in practice or outside of clinical trials. The novel Simplified Endoscopic Mucosal Assessment for Crohn's Disease (SEMA-CD) was developed to be easy to use in routine clinical practice and as a trial end point. We conducted a study to assess and validate the reliability and feasibility of SEMA-CD as a measure of endoscopic disease activity.
Methods: Pre- and post-treatment ileocolonoscopy videos of pediatric (n = 36) and adult (n = 74) CD patients from 2 ustekinumab clinical trials were each scored with SEMA-CD by 2 to 3 professional central readers, blinded to clinical history and other video scorings; the correlation between SEMA-CD and SES-CD previously completed during the trials was assessed. Sensitivity to change, inter- and intrarater reliability, and comparative ease of scoring were also assessed.
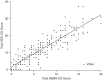
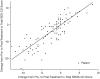
Results: The SEMA-CD strongly correlated with SES-CD (Spearman ρ = 0.89; 95% confidence interval, 0.86-0.92). Pre- to post-treatment changes in SEMA-CD vs in SES-CD were strongly correlated, and the correlation remained strong between the scores when compared by study population (pediatric, adult), disease severity, and video quality. Intra- and inter-rater reliability were good, and SEMA-CD was rated easier than SES-CD to score 63.0% of the time, although slightly more difficult than SES-CD to score <1.0% of the time.
Conclusions: The SEMA-CD is reliable, reproducible, sensitive to change, and easy to use in both pediatric and adult patients with CD.
Keywords: Crohn’s disease; SEMA-CD; SES-CD; endoscopic disease activity; validation.
© 2022 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
J.A. has received research grants/funding from Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson).
T.M.B. has acted in a consultancy role for Medtronic, Boston Scientific, Docbot AI, and Wision AI.
A.S.C. has acted in a consultancy role for Abbvie, Arena, Artizan (SAB), Artugen, Bacainn, BMS, Equillium, Grifols, Janssen, Pfizer, Procise, Prometheus (SAB), Samsung, and Takeda.
R.B.C. has acted in a consultancy/advisory role for Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson).
L.S.C. is an employee of Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson) and owns Johnson & Johnson stock and/or stock options.
T.C.H. is an employee of Immunology Global Medical Affairs, Janssen Pharmaceutical Companies (a subsidiary of Johnson & Johnson) and owns Johnson & Johnson stock and/or stock options.
C.S.H. has no disclosures to report.
B.L. has no disclosures to report.
K.H.L. is an employee of Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson) and owns Johnson & Johnson stock and/or stock options.
D.S.M. owns GI Reviewers, LLC.
L.N. is an employee of Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson) and owns Johnson & Johnson stock and/or stock options.
S.V. is an employee of Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson) and owns Johnson & Johnson stock and/or stock options.
Y.X. is an employee of Cytel, Inc., Waltham, MA, USA, and has acted in a consultancy/advisory role for Janssen Research & Development, LLC.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- United States Food and Drug Administration. Real-world data: assessing electronic health records and medical claims data to support regulatory decision-making for drug and biological products. September 2021, Accessed November 30, 2021, https://www.fda.gov/media/152503/download - PMC - PubMed
-
- European Medicines Agency. Guideline on registry-based studies. 22 October 2021, Accessed November 2, 2021, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-re...
-
- Abreu MT, Sandborn WJ.. IOIBD Defining Endpoints and Biomarkers in Inflammatory Bowel Disease Writing Group. Defining endpoints and biomarkers in inflammatory bowel disease: moving the needle through clinical trial design. Gastroenterology 2020;159(6):2013–2018.e7. - PubMed
-
- European Medicines Agency. Guideline on the development of new medicinal products for the treatment of Crohn’s disease. 28 June 2018, Accessed October 12, 2021, https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guidel...
-
- Walsh AJ, Bryant RV, Travis SP.. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13(10):567–579. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

