Preparation and application of a specific single-chain variable fragment against artemether
- PMID: 36049377
- PMCID: PMC9764824
- DOI: 10.1016/j.jpba.2022.115020
Preparation and application of a specific single-chain variable fragment against artemether
Abstract
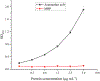
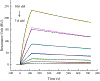
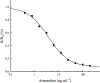
Artemether, an artemisinin derivative, is a component of the commonly used artemisinin-based combination therapy, artemether-lumefantrine. In this study, we cloned the VH and VL genes of a cell line (mAb 2G12E1) producing a monoclonal antibody specific to artemether, and used to construct a recombinant DNA of single-chain variable fragment (scFv). The scFv was constructed into prokaryotic expression vectors pET32a (+), pET22b (+), pGEX-2T, and pMAL-p5x, respectively. However, only the pMAL-p5x/scFv could be induced to express soluble scFv with comparable sensitivity and specificity to that of mAb 2G12E1. Based on the anti-artemether scFv, an indirect competitive enzyme-linked immunosorbent assay (icELISA) was developed. The 50% of inhibition concentration (IC50) value and the working range based on IC20 to IC80 were 4.33 ng mL-1 and 1.05-22.65 ng mL-1, respectively. The artemether content in different drugs were determined by the developed icELISA, and the results were consistent to those determined by ultra performance liquid chromatography (UPLC). The anti-artemether scFv prepared in the current study could be a valuable genetically engineered antibody applied for artemether monitoring and specific binding mechanism studying.
Keywords: Artemether; Artemisinin-based combination therapies (ACTs); ELISA; Malaria; scFv.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Development of a specific monoclonal antibody-based ELISA to measure the artemether content of antimalarial drugs.PLoS One. 2013 Nov 13;8(11):e79154. doi: 10.1371/journal.pone.0079154. eCollection 2013. PLoS One. 2013. PMID: 24236102 Free PMC article.
-
Preparation of a single-chain variable fragment and a recombinant antigen-binding fragment against the anti-malarial drugs, artemisinin and artesunate, and their application in an ELISA.Anal Chem. 2012 Feb 21;84(4):2002-8. doi: 10.1021/ac203131f. Epub 2012 Feb 7. Anal Chem. 2012. PMID: 22260329
-
Comparison of single-chain variable fragments and monoclonal antibody against dihydroartemisinin.J Immunol Methods. 2024 Sep;532:113728. doi: 10.1016/j.jim.2024.113728. Epub 2024 Jul 24. J Immunol Methods. 2024. PMID: 39059746
-
[Combined antimalarial therapy using artemisinin].Parassitologia. 2004 Jun;46(1-2):85-7. Parassitologia. 2004. PMID: 15305693 Review. Italian.
-
Quantitative determination of the antimalarials artemether and lumefantrine in biological samples: A review.J Pharm Biomed Anal. 2019 Feb 20;165:304-314. doi: 10.1016/j.jpba.2018.12.021. Epub 2018 Dec 13. J Pharm Biomed Anal. 2019. PMID: 30579231 Review.
References
-
- World Health Organization, world malaria report 2021. https://www.who.int/publications/i/item/9789240040496, 2021. (Accessed 29 March 2022).
-
- World Health Organization, WHO Guidelines for malaria. https://app.magicapp.org/#/guideline/j9QMBj/section/nBMKdz, 2022. (Accessed 29 March 2022).
-
- World Health Organization, Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). https://www.who.int/publications/i/item/9789240012813, 2020. (Accessed 29 March 2022).
-
- Lamy MCM, Framing the challenge of poor-quality medicines: problem definition and policy making in Cambodia, Laos, and Thailand, London School of Hygiene & Tropical Medicine, 2017. DOI: 10.17037/PUBS.04645490. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

