Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials
- PMID: 36049498
- PMCID: PMC7613583
- DOI: 10.1016/S0140-6736(22)01545-8
Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials
Erratum in
-
Department of Error.Lancet. 2022 Oct 8;400(10359):1194. doi: 10.1016/S0140-6736(22)01891-8. Lancet. 2022. PMID: 36216002 Free PMC article. No abstract available.
Abstract
Background: Statin therapy is effective for the prevention of atherosclerotic cardiovascular disease and is widely prescribed, but there are persisting concerns that statin therapy might frequently cause muscle pain or weakness. We aimed to address these through an individual participant data meta-analysis of all recorded adverse muscle events in large, long-term, randomised, double-blind trials of statin therapy.
Methods: Randomised trials of statin therapy were eligible if they aimed to recruit at least 1000 participants with a scheduled treatment duration of at least 2 years, and involved a double-blind comparison of statin versus placebo or of a more intensive versus a less intensive statin regimen. We analysed individual participant data from 19 double-blind trials of statin versus placebo (n=123 940) and four double-blind trials of a more intensive versus a less intensive statin regimen (n=30 724). Standard inverse-variance-weighted meta-analyses of the effects on muscle outcomes were conducted according to a prespecified protocol.
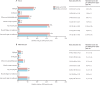
Findings: Among 19 placebo-controlled trials (mean age 63 years [SD 8], with 34 533 [27·9%] women, 59 610 [48·1%] participants with previous vascular disease, and 22 925 [18·5%] participants with diabetes), during a weighted average median follow-up of 4·3 years, 16 835 (27·1%) allocated statin versus 16 446 (26·6%) allocated placebo reported muscle pain or weakness (rate ratio [RR] 1·03; 95% CI 1·01-1·06). During year 1, statin therapy produced a 7% relative increase in muscle pain or weakness (1·07; 1·04-1·10), corresponding to an absolute excess rate of 11 (6-16) events per 1000 person-years, which indicates that only one in 15 ([1·07-1·00]/1·07) of these muscle-related reports by participants allocated to statin therapy were actually due to the statin. After year 1, there was no significant excess in first reports of muscle pain or weakness (0·99; 0·96-1·02). For all years combined, more intensive statin regimens (ie, 40-80 mg atorvastatin or 20-40 mg rosuvastatin once per day) yielded a higher RR than less intensive or moderate-intensity regimens (1·08 [1·04-1·13] vs 1·03 [1·00-1·05]) compared with placebo, and a small excess was present (1·05 [0·99-1·12]) for more intensive regimens after year 1. There was no clear evidence that the RR differed for different statins, or in different clinical circumstances. Statin therapy yielded a small, clinically insignificant increase in median creatine kinase values of approximately 0·02 times the upper limit of normal.
Interpretation: Statin therapy caused a small excess of mostly mild muscle pain. Most (>90%) of all reports of muscle symptoms by participants allocated statin therapy were not due to the statin. The small risks of muscle symptoms are much lower than the known cardiovascular benefits. There is a need to review the clinical management of muscle symptoms in patients taking a statin.
Funding: British Heart Foundation, Medical Research Council, Australian National Health and Medical Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests JA and DP report receiving a grant to their research institution from Novartis for the ORION 4 trial of inclisiran. AK reports receiving grants for his institution from Abbott, Amgen, and Mylan; consulting fees from AstraZeneca; honoraria from Sanofi and Pfizer; and is a Data Safety Monitoring Board member of the Kowa PROMINENT trial. JS reports receiving grants for his institution from Abbvie, Astra Zeneca, Bayer, Bristol Myers Squibb, Pfizer, and Roche. RC reports a patent for a statin-related myopathy genetic test licensed to University of Oxford from Boston Heart Diagnostics (RC has waived any personal reward). All other authors declare no competing interests.
Figures



Comment in
-
Statin intolerance: time to stop letting it get in the way of treating patients.Lancet. 2022 Sep 10;400(10355):791-793. doi: 10.1016/S0140-6736(22)01643-9. Epub 2022 Aug 29. Lancet. 2022. PMID: 36049497 No abstract available.
-
Cardiovascular benefits of statins outweigh small risk of muscle pain.Nat Rev Cardiol. 2022 Nov;19(11):721. doi: 10.1038/s41569-022-00785-8. Nat Rev Cardiol. 2022. PMID: 36127460 No abstract available.
-
Solid evidence that very few muscle symptoms are due to statin therapy.Eur Heart J. 2023 Jan 1;44(1):12-13. doi: 10.1093/eurheartj/ehac620. Eur Heart J. 2023. PMID: 36342313 No abstract available.
-
Statins increase muscle pain or weakness at 1 y, with an absolute excess of 11 events/1000 person-y.Ann Intern Med. 2023 Jan;176(1):JC4. doi: 10.7326/J22-0101. Epub 2023 Jan 3. Ann Intern Med. 2023. PMID: 36592471
References
-
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. - PubMed
-
- Cholesterol Treatment Trialists' Collaboration Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

