SARS-CoV-2-specific T cell responses in patients with multisystem inflammatory syndrome in children
- PMID: 36049601
- PMCID: PMC9423880
- DOI: 10.1016/j.clim.2022.109106
SARS-CoV-2-specific T cell responses in patients with multisystem inflammatory syndrome in children
Abstract
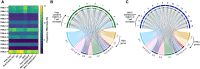
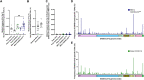
Multisystem inflammatory syndrome in children (MIS-C) is a severe complication of SARS-CoV-2 infections that occurs in the pediatric population. We sought to characterize T cell responses in MIS-C compared to COVID-19 and pediatric hyperinflammatory syndromes. MIS-C was distinct from COVID-19 and hyperinflammatory syndromes due to an expansion of T cells expressing TRBV11-2 that was not associated with HLA genotype. Children diagnosed with MIS-C, but who were negative for SARS-CoV-2 by PCR and serology, did not display Vβ skewing. There was no difference in the proportion of T cells that became activated after stimulation with SARS-CoV-2 peptides in children with MIS-C compared to convalescent COVID-19. The frequency of SARS-CoV-2-specific TCRs and the antigens recognized by these TCRs were comparable in MIS-C and COVID-19. Expansion of Vβ11-2+ T cells was a specific biomarker of MIS-C patients with laboratory confirmed SARS-CoV-2 infections. Children with MIS-C had robust antigen-specific T cell responses to SARS-CoV-2.
Keywords: Coronavirus disease 2019 (COVID-19); Multisystem inflammatory syndrome in children (MIS-C); Pediatrics; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); T cell receptor (TCR) repertoire.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest LAH has received salary support from CARRA; consulting fees from Sobi, Pfizer, and Adaptive Biotechnologies; and investigator-initiated research grants from Bristol-Myers Squibb.
Figures




Similar articles
-
HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children.J Clin Invest. 2021 May 17;131(10):e146614. doi: 10.1172/JCI146614. J Clin Invest. 2021. PMID: 33705359 Free PMC article.
-
Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis.Front Immunol. 2022 Jul 7;13:941009. doi: 10.3389/fimmu.2022.941009. eCollection 2022. Front Immunol. 2022. PMID: 35874696 Free PMC article.
-
SARS-CoV-2-Specific T Cell Responses Are Stronger in Children With Multisystem Inflammatory Syndrome Compared to Children With Uncomplicated SARS-CoV-2 Infection.Front Immunol. 2022 Jan 18;12:793197. doi: 10.3389/fimmu.2021.793197. eCollection 2021. Front Immunol. 2022. PMID: 35116027 Free PMC article.
-
Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence.Int J Mol Sci. 2023 Mar 16;24(6):5711. doi: 10.3390/ijms24065711. Int J Mol Sci. 2023. PMID: 36982783 Free PMC article. Review.
-
Multisystem Inflammatory Syndrome in Children (MIS-C).Curr Allergy Asthma Rep. 2022 May;22(5):53-60. doi: 10.1007/s11882-022-01031-4. Epub 2022 Mar 22. Curr Allergy Asthma Rep. 2022. PMID: 35314921 Free PMC article. Review.
Cited by
-
COVID-19-Related Multi-systemic Inflammatory Syndrome in Children (MIS-C).Adv Exp Med Biol. 2024;1448:409-425. doi: 10.1007/978-3-031-59815-9_28. Adv Exp Med Biol. 2024. PMID: 39117830 Review.
-
Human genetic and immunological determinants of SARS-CoV-2 infection and multisystem inflammatory syndrome in children.Clin Exp Immunol. 2025 Jan 21;219(1):uxae062. doi: 10.1093/cei/uxae062. Clin Exp Immunol. 2025. PMID: 39028583 Free PMC article. Review.
-
Autoimmunity and Immunodeficiency in Severe SARS-CoV-2 Infection and Prolonged COVID-19.Curr Issues Mol Biol. 2022 Dec 21;45(1):33-50. doi: 10.3390/cimb45010003. Curr Issues Mol Biol. 2022. PMID: 36661489 Free PMC article. Review.
-
Label-Free Proteomics of Severe Acute Hepatitis of Unknown Origin in Children by High-Resolution Mass Spectrometry.ACS Omega. 2024 Dec 13;9(51):50685-50694. doi: 10.1021/acsomega.4c08745. eCollection 2024 Dec 24. ACS Omega. 2024. PMID: 39741806 Free PMC article.
-
SARS-CoV-2 infection and recovery in children: Distinct T cell responses in MIS-C compared to COVID-19.J Exp Med. 2023 Aug 7;220(8):e20221518. doi: 10.1084/jem.20221518. Epub 2023 May 3. J Exp Med. 2023. PMID: 37133746 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

