SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals
- PMID: 36050304
- PMCID: PMC9435409
- DOI: 10.1038/s41467-022-32389-8
SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals
Abstract
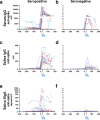
Immune responses at the respiratory mucosal interface are critical to prevent respiratory infections but it is unclear to what extent antigen specific mucosal secretory IgA (SIgA) antibodies are induced by mRNA vaccination in humans. Here we analyze paired serum and saliva samples from patients with and without prior coronavirus disease 2019 (COVID-19) at multiple time points pre and post severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination. Our results suggest mucosal SIgA responses induced by mRNA vaccination are impacted by pre-existing immunity. Indeed, vaccination induced a minimal mucosal SIgA response in individuals without pre-exposure to SARS-CoV-2 while SIgA induction after vaccination was more efficient in patients with a history of COVID-19.
© 2022. The Author(s).
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines which list F.K. as co-inventor. V.S. is also listed on the serological assay patent application as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for Merck and Pfizer (before 2020) and is currently consulting for Pfizer, Seqirus, Third Rock Ventures, and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. The remaining authors declare no competing interests.
Figures

References
-
- WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Datahttps://covid19.who.int/ (WHO, accessed 13 July 2022).
-
- Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 1989;146:107–116. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

