Differential pharmacological and sex-specific effects of antimuscarinic agents at the hypoglossal motor nucleus in vivo in rats
- PMID: 36050440
- PMCID: PMC9437041
- DOI: 10.1038/s41598-022-19233-1
Differential pharmacological and sex-specific effects of antimuscarinic agents at the hypoglossal motor nucleus in vivo in rats
Abstract
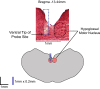
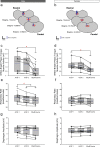
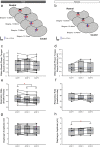
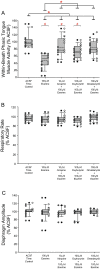
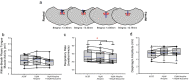
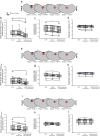
Successful cholinergic-noradrenergic pharmacotherapy for obstructive sleep apnea (OSA) is thought to be due to effects at the hypoglossal motor nucleus (HMN). Clinical efficacy varies with muscarinic-receptor (MR) subtype affinities. We hypothesized that oxybutynin (cholinergic agent in successful OSA pharmacotherapy) is an effective MR antagonist at the HMN and characterized its efficacy with other antagonists. We recorded tongue muscle activity of isoflurane anesthetized rats (121 males and 60 females, 7-13 per group across 13 protocols) in response to HMN microperfusion with MR antagonists with and without: (i) eserine-induced increased endogenous acetylcholine at the HMN and (ii) muscarine. Eserine-induced increased acetylcholine decreased tongue motor activity (p < 0.001) with lesser cholinergic suppression in females versus males (p = 0.017). Motor suppression was significantly attenuated by the MR antagonists atropine, oxybutynin, and omadacycline (MR2 antagonist), each p < 0.001, with similar residual activity between agents (p ≥ 0.089) suggesting similar efficacy at the HMN. Sex differences remained with atropine and oxybutynin (p < 0.001 to 0.05) but not omadacycline (p = 0.722). Muscarine at the HMN also decreased motor activity (p < 0.001) but this was not sex-specific (p = 0.849). These findings have translational relevance to antimuscarinic agents in OSA pharmacotherapy and understanding potential sex differences in HMN suppression with increased endogenous acetylcholine related to sparing nicotinic excitation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Similar articles
-
Opposing muscarinic and nicotinic modulation of hypoglossal motor output to genioglossus muscle in rats in vivo.J Physiol. 2005 Jun 15;565(Pt 3):965-80. doi: 10.1113/jphysiol.2005.084657. Epub 2005 Apr 7. J Physiol. 2005. PMID: 15817635 Free PMC article.
-
Modulation of TASK-1/3 channels at the hypoglossal motoneuron pool and effects on tongue motor output and responses to excitatory inputs in vivo: implications for strategies for obstructive sleep apnea pharmacotherapy.Sleep. 2021 Jan 21;44(1):zsaa144. doi: 10.1093/sleep/zsaa144. Sleep. 2021. PMID: 32745213 Free PMC article.
-
Opioid receptor mechanisms at the hypoglossal motor pool and effects on tongue muscle activity in vivo.J Physiol. 2009 Jun 1;587(Pt 11):2677-92. doi: 10.1113/jphysiol.2009.171678. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403616 Free PMC article.
-
Activation of the Hypoglossal to Tongue Musculature Motor Pathway by Remote Control.Sci Rep. 2017 Apr 6;7:45860. doi: 10.1038/srep45860. Sci Rep. 2017. PMID: 28383527 Free PMC article.
-
Presynaptic muscarinic (M3) receptors reduce excitatory transmission in dopamine neurons of the rat mesencephalon.Neuroscience. 1999;91(2):557-65. doi: 10.1016/s0306-4522(98)00619-8. Neuroscience. 1999. PMID: 10366013
Cited by
-
Cholinergic modulation of upper airway control: maturational changes and mechanisms at cellular and synaptic levels.J Neurophysiol. 2025 Jan 1;133(1):46-59. doi: 10.1152/jn.00165.2024. Epub 2024 Nov 28. J Neurophysiol. 2025. PMID: 39607299 Free PMC article. Review.
-
A muscarinic, GIRK channel-mediated inhibition of inspiratory-related XII nerve motor output emerges in early postnatal development in mice.J Appl Physiol (1985). 2023 Nov 1;135(5):1041-1052. doi: 10.1152/japplphysiol.00042.2023. Epub 2023 Sep 28. J Appl Physiol (1985). 2023. PMID: 37767557 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

