Urinary marker panels for aggressive prostate cancer detection
- PMID: 36050450
- PMCID: PMC9437030
- DOI: 10.1038/s41598-022-19134-3
Urinary marker panels for aggressive prostate cancer detection
Abstract
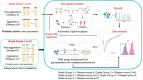
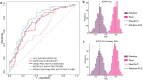
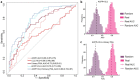
Majority of patients with indolent prostate cancer (PCa) can be managed with active surveillance. Therefore, finding biomarkers for classifying patients between indolent and aggressive PCa is essential. In this study, we investigated urinary marker panels composed of urinary glycopeptides and/or urinary prostate-specific antigen (PSA) for their clinical utility in distinguishing non-aggressive (Grade Group 1) from aggressive (Grade Group ≥ 2) PCa. Urinary glycopeptides acquired via data-independent acquisition mass spectrometry (DIA-MS) were quantitatively analyzed, where prostatic acid phosphatase (ACPP), clusterin (CLU), alpha-1-acid glycoprotein 1 (ORM1), and CD antigen 97 (CD97) were selected to be evaluated in various combinations with and without urinary PSA. Targeted parallel reaction monitoring (PRM) assays of the glycopeptides from urinary ACPP and CLU were investigated along with urinary PSA for the ability of aggressive PCa detection. The multi-urinary marker panels, combined via logistic regression, were statistically evaluated using bootstrap resampling and validated by an independent cohort. Majority of the multi-urinary marker panels (e.g., a panel consisted of ACPP, CLU, and Urinary PSA) achieved area under the curve (AUC) ranged from 0.70 to 0.85. Thus, multi-marker panels investigated in this study showed clinically meaningful results on aggressive PCa detection to separate Grade Group 1 from Grade Group 2 and above warranting further evaluation in clinical setting in future.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, Doubeni CA, Ebell M, Epling JW, Kemper AR, Krist AH, Kubik M, Seth Landefeld C, Mangione CM, Silverstein M, Simon MA, Siu AL, Tseng C-W. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319:1901–1913. doi: 10.1001/jama.2018.0161. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

