Sphingosine kinase 1 promotes tumor immune evasion by regulating the MTA3-PD-L1 axis
- PMID: 36050478
- PMCID: PMC9508236
- DOI: 10.1038/s41423-022-00911-z
Sphingosine kinase 1 promotes tumor immune evasion by regulating the MTA3-PD-L1 axis
Abstract
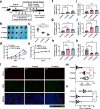
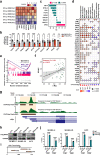
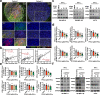
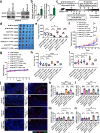
Immune checkpoint blockade (ICB) exhibits considerable benefits in malignancies, but its overall response rate is limited. Previous studies have shown that sphingosine kinases (SPHKs) are critical in the tumor microenvironment (TME), but their role in immunotherapy is unclear. We performed integrative analyses including bioinformatics analysis, functional study, and clinical validation to investigate the role of SPHK1 in tumor immunity. Functionally, we demonstrated that the inhibition of SPHK1 significantly suppressed tumor growth by promoting antitumor immunity in immunocompetent melanoma mouse models and tumor T-cell cocultures. A mechanistic analysis revealed that MTA3 functions as the downstream target of SPHK1 in transcriptionally regulating tumor PD-L1. Preclinically, we found that anti-PD-1 monoclonal antibody (mAb) treatment significantly rescued tumor SPHK1 overexpression or tumor MTA3 overexpression-mediated immune evasion. Significantly, we identified SPHK1 and MTA3 as biological markers for predicting the efficacy of anti-PD-1 mAb therapy in melanoma patients. Our findings revealed a novel role for SPHK1 in tumor evasion mediated by regulating the MTA3-PD-L1 axis, identified SPHK1 and MTA3 as predictors for assessing the efficacy of PD-1 mAb treatment, and provided a therapeutic possibility for the treatment of melanoma patients.
Keywords: Immune checkpoint blockade; Melanoma; Programmed cell death ligand 1; Programmed cell death protein 1; Sphingosine kinase; Tumor microenvironment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
SPHK1 promotes HNSCC immune evasion by regulating the MMP1-PD-L1 axis.Theranostics. 2024 Oct 28;14(18):7199-7218. doi: 10.7150/thno.102390. eCollection 2024. Theranostics. 2024. PMID: 39629135 Free PMC article.
-
eEF2K promotes PD-L1 stabilization through inactivating GSK3β in melanoma.J Immunother Cancer. 2022 Mar;10(3):e004026. doi: 10.1136/jitc-2021-004026. J Immunother Cancer. 2022. PMID: 35347072 Free PMC article.
-
ANXA1-derived peptide for targeting PD-L1 degradation inhibits tumor immune evasion in multiple cancers.J Immunother Cancer. 2023 Mar;11(3):e006345. doi: 10.1136/jitc-2022-006345. J Immunother Cancer. 2023. PMID: 37001908 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Immune Checkpoint Blockade in Cancer Immunotherapy: Mechanisms, Clinical Outcomes, and Safety Profiles of PD-1/PD-L1 Inhibitors.Arch Immunol Ther Exp (Warsz). 2020 Nov 13;68(6):36. doi: 10.1007/s00005-020-00601-6. Arch Immunol Ther Exp (Warsz). 2020. PMID: 33185750 Review.
Cited by
-
Prognostic and immunological significance of metastasis-associated protein 3 in patients with thymic epithelial tumors.Discov Oncol. 2024 Jun 9;15(1):216. doi: 10.1007/s12672-024-01066-1. Discov Oncol. 2024. PMID: 38852126 Free PMC article.
-
Immunosuppressive Effects of Multiple Myeloma-Derived Extracellular Vesicles Through T Cell Exhaustion.Cancer Sci. 2025 Jul;116(7):1861-1870. doi: 10.1111/cas.70099. Epub 2025 May 8. Cancer Sci. 2025. PMID: 40344445 Free PMC article.
-
Upregulated PARP1 confers breast cancer resistance to CDK4/6 inhibitors via YB-1 phosphorylation.Exp Hematol Oncol. 2023 Nov 30;12(1):100. doi: 10.1186/s40164-023-00462-7. Exp Hematol Oncol. 2023. PMID: 38037159 Free PMC article.
-
Sphingolipid metabolism and regulated cell death in malignant melanoma.Apoptosis. 2024 Dec;29(11-12):1860-1878. doi: 10.1007/s10495-024-02002-y. Epub 2024 Jul 28. Apoptosis. 2024. PMID: 39068623 Review.
-
SPHK1 promotes HNSCC immune evasion by regulating the MMP1-PD-L1 axis.Theranostics. 2024 Oct 28;14(18):7199-7218. doi: 10.7150/thno.102390. eCollection 2024. Theranostics. 2024. PMID: 39629135 Free PMC article.
References
-
- American Cancer Society. Cancer facts & figures 2020. https://www.cancer.org/research/cancer-facts-statistics.html 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

