Mulberry Diels-Alder-type adducts: isolation, structure, bioactivity, and synthesis
- PMID: 36050566
- PMCID: PMC9436459
- DOI: 10.1007/s13659-022-00355-y
Mulberry Diels-Alder-type adducts: isolation, structure, bioactivity, and synthesis
Abstract
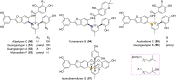
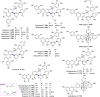
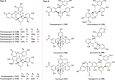
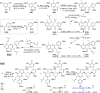
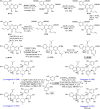
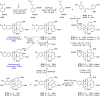
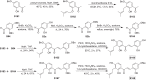
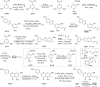
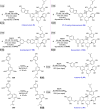
Mulberry Diels-Alder-type adducts (MDAAs) are unique phenolic natural products biosynthetically derived from the intermolecular [4 + 2]-cycloaddition of dienophiles (mainly chalcones) and dehydroprenylphenol dienes, which are exclusively distributed in moraceous plants. A total of 166 MDAAs with diverse skeletons have been isolated and identified since 1980. Structurally, the classic MDAAs characterized by the chalcone-skeleton dienophiles can be divided into eight groups (Types A - H), while others with non-chalcone dienophiles or some variations of classic MDAAs are non-classic MDAAs (Type I). These compounds have attracted significant attention of natural products and synthetic chemists due to their complex architectures, remarkable biological activities, and synthetic challenges. The present review provides a comprehensive summary of the structural properties, bioactivities, and syntheses of MDAAs. Cited references were collected between 1980 and 2021 from the SciFinder, Web of Science, and China National Knowledge Internet (CNKI).
Keywords: Bioactivity; MDAAs; Mulberry Diels–Alder-type adducts; Natural products; Synthesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
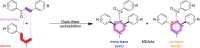







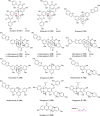





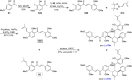


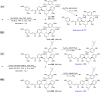
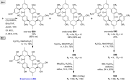



References
-
- Nomura T, Fukai T, Kuwanon G. a new flavone derivative from the root barks of the cultivated mulberry tree (Morus alba L) Chem Pharm Bull. 1980;28:2548–2552. doi: 10.1248/cpb.28.2548. - DOI
-
- Nomura T, Fukai T, Narita T. Hypotensive constituent, kuwanon H, a new flavone derivative from the root bark of the cultivated mulberry tree (Morus alba L) Heterocycles. 1980;14:1943–1951. doi: 10.3987/R-1980-12-1943. - DOI
-
- Takasugi M, Ishikawa S-I, Nagao S, Masamune T, Shirata A, Takahashi K. Albanins F and G, natural Diels-Alder adducts from mulberry. Chem Lett. 1980;9:1577–1580. doi: 10.1246/cl.1980.1577. - DOI
-
- Takasugi M, Nagao S, Masamune T, Shirata A, Takahashi K. Chalcomoracin, a natural Diels-Alder adduct from diseased mulberry. Chem Lett. 1980;9:1573–1576. doi: 10.1246/cl.1980.1573. - DOI
-
- Nomura T, Fukai T, Narita T, Terada S, Uzawa J, Iitaka Y, Takasugi M, Ishikawa S-I, Nagao S, Masamune T. Confirmation of the structures of kuwanons G and H (albanins F and G) by partial synthesis. Tetrahedron Lett. 1981;22:2195–2198. doi: 10.1016/S0040-4039(01)90496-4. - DOI
Publication types
Grants and funding
- 81973203/National Natural Science Foundation of China
- 81973195/National Natural Science Foundation of China
- 2020A1515010841/Guangdong Basic and Applied Basic Research Foundation, China
- SZBL2021080601007/the Open Program of Shenzhen Bay Laboratory
- SML2021SP301/the Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

