Emergency contraception subsidy in Canada: a comparative policy analysis
- PMID: 36050668
- PMCID: PMC9438154
- DOI: 10.1186/s12913-022-08416-1
Emergency contraception subsidy in Canada: a comparative policy analysis
Abstract
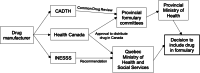
Background: In Canada, cost prohibits access to emergency contraception (EC) which may assist to prevent unintended pregnancy. The drug, ulipristal acetate (UPA-EC), is more clinically effective and cost-effective than the prior standard levonorgestrel (LNG-EC). We analyzed provincial EC subsidization policies and examined underlying decision-making processes.
Methods: We undertook documentary analysis of provincial EC subsidization policies in publicly available drug formularies. We conducted semi-structured interviews with key informants to explore the processes underlying current policies.
Results: Quebec is the only province to subsidize UPA-EC, whilst all ten provinces subsidize LNG-EC. As such, provincial EC subsidization policies do not align with the latest UPA-EC evidence. Interviews revealed that evidence was valued in the policymaking process and formulary decisions were made through interdisciplinary consensus.
Conclusions: We identify a gap between EC subsidization policies and the latest evidence. Institutional structures affect policies reflecting evolving evidence. Increasing interdisciplinary mechanisms may encourage evidence-based policies.
Keywords: Canada; Emergency contraception; Evidence-based policy; Health policy; Ulipristal acetate; Universal subsidy.
© 2022. The Author(s).
Conflict of interest statement
There were no financial or non-financial competing interests for either author.
Similar articles
-
Canadian Contraception Consensus (Part 2 of 4).J Obstet Gynaecol Can. 2015 Nov;37(11):1033-9. doi: 10.1016/s1701-2163(16)30054-8. J Obstet Gynaecol Can. 2015. PMID: 26629725 English, French.
-
Canadian Contraception Consensus (Part 1 of 4).J Obstet Gynaecol Can. 2015 Oct;37(10):936-42. doi: 10.1016/s1701-2163(16)30033-0. J Obstet Gynaecol Can. 2015. PMID: 26606712 English, French.
-
Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception.Clin Ther. 2012 Jan;34(1):24-36. doi: 10.1016/j.clinthera.2011.11.012. Epub 2011 Dec 9. Clin Ther. 2012. PMID: 22154199 Review.
-
Prevention of unintended pregnancies by using emergency contraception: the differences between levonorgestrel and ulipristal acetate. A theoretical model using data from a survey on the use of emergency contraception in Spain, 2017.Gynecol Endocrinol. 2019 Jul;35(7):582-585. doi: 10.1080/09513590.2018.1559286. Epub 2019 Jan 7. Gynecol Endocrinol. 2019. PMID: 30614295
-
The price of emergency contraception in the United States: what is the cost-effectiveness of ulipristal acetate versus single-dose levonorgestrel?Contraception. 2013 Mar;87(3):385-90. doi: 10.1016/j.contraception.2012.08.034. Epub 2012 Oct 4. Contraception. 2013. PMID: 23040122
References
-
- FSRH Clinical Effectiveness Unit. FSRH Guideline: Emergency contraception. 2017.
-
- Black A, Guilbert E, Costecu D, Dunn S, Fisher W, Kives S, et al. Canadian Contraception Consensus (Part 1 of 4). J Obstet Gynaecol Can \ Journal d’obstétrique et gynécologie du Canada. 2015;37:936–42. - PubMed
-
- Black A, Guilbert E, Costecu D, Dunn S, Fisher W, Kives S, et al. Canadian Contraception Consensus Chapter 3 Emergency Contraception. Canadian Contraception Consensus (Part 1 of 4). 2015;37:S20–8.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous