Control of redox potential in a novel continuous bioelectrochemical system led to remarkable metabolic and energetic responses of Clostridium pasteurianum grown on glycerol
- PMID: 36050762
- PMCID: PMC9434860
- DOI: 10.1186/s12934-022-01902-5
Control of redox potential in a novel continuous bioelectrochemical system led to remarkable metabolic and energetic responses of Clostridium pasteurianum grown on glycerol
Abstract
Background: Electro-fermentation (EF) is an emerging tool for bioprocess intensification. Benefits are especially expected for bioprocesses in which the cells are enabled to exchange electrons with electrode surfaces directly. It has also been demonstrated that the use of electrical energy in BES can increase bioprocess performance by indirect secondary effects. In this case, the electricity is used to alter process parameters and indirectly activate desired pathways. In many bioprocesses, oxidation-reduction potential (ORP) is a crucial process parameter. While C. pasteurianum fermentation of glycerol has been shown to be significantly influenced electrochemically, the underlying mechanisms are not clear. To this end, we developed a system for the electrochemical control of ORP in continuous culture to quantitatively study the effects of ORP alteration on C. pasteurianum by metabolic flux analysis (MFA), targeted metabolomics, sensitivity and regulation analysis.
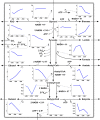
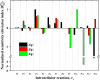
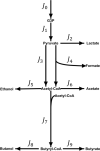
Results: In the ORP range of -462 mV to -250 mV, the developed algorithm enabled a stable anodic electrochemical control of ORP at desired set-points and a fixed dilution rate of 0.1 h-1. An overall increase of 57% in the molar yield for 1,3-propanediol was observed by an ORP increase from -462 to -250 mV. MFA suggests that C. pasteurianum possesses and uses cellular energy generation mechanisms in addition to substrate-level phosphorylation. The sensitivity analysis showed that ORP exerted its strongest impact on the reaction of pyruvate-ferredoxin-oxidoreductase. The regulation analysis revealed that this influence is mainly of a direct nature. Hence, the observed metabolic shifts are primarily caused by direct inhibition of the enzyme upon electrochemical production of oxygen. A similar effect was observed for the enzyme pyruvate-formate-lyase at elevated ORP levels.
Conclusions: The results show that electrochemical ORP alteration is a suitable tool to steer the metabolism of C. pasteurianum and increase product yield for 1,3-propanediol in continuous culture. The approach might also be useful for application with further anaerobic or anoxic bioprocesses. However, to maximize the technique's efficiency, it is essential to understand the chemistry behind the ORP change and how the microbial system responds to it by transmitted or direct effects.
Keywords: BES; Clostridium pasteurianum; Continuous fermentation; ORP; Redox metabolism; Regulation analysis; Symbolic metabolic control analysis.
© 2022. The Author(s).
Conflict of interest statement
Not applicable.
Figures




References
-
- Kjaergaard L. Advances in Biochemical Engineering. Berlin/Heidelberg: Springer-Verlag; 1977. The redox potential: Its use and control in biotechnology; pp. 131–150.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

