Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction
- PMID: 36050763
- PMCID: PMC9438179
- DOI: 10.1186/s12933-022-01604-7
Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction
Abstract
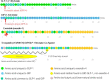
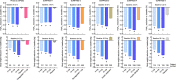
Tirzepatide is the first dual GIP/GLP-1 receptor co-agonist approved for the treatment of type 2 diabetes in the USA, Europe, and the UAE. Tirzepatide is an acylated peptide engineered to activate the GIP and GLP-1 receptors, key mediators of insulin secretion that are also expressed in regions of the brain that regulate food intake. Five clinical trials in type 2-diabetic subjects (SURPASS 1-5) have shown that tirzepatide at 5-15 mg per week reduces both HbA1c (1.24 to 2.58%) and body weight (5.4-11.7 kg) by amounts unprecedented for a single agent. A sizable proportion of patients (23.0 to 62.4%) reached an HbA1c of < 5.7% (which is the upper limit of the normal range indicating normoglycaemia), and 20.7 to 68.4% lost more than 10% of their baseline body weight. Tirzepatide was significantly more effective in reducing HbA1c and body weight than the selective GLP-1 RA semaglutide (1.0 mg per week), and titrated basal insulin. Adverse events related to tirzepatide were similar to what has been reported for selective GLP-1RA, mainly nausea, vomiting, diarrhoea, and constipation, that were more common at higher doses. Cardiovascular events have been adjudicated across the whole study program, and MACE-4 (nonfatal myocardial infarction, non-fatal stroke, cardiovascular death and hospital admission for angina) events tended to be reduced over up to a 2 year-period, albeit with low numbers of events. For none of the cardiovascular events analysed (MACE-4, or its components) was a hazard ratio > 1.0 vs. pooled comparators found in a meta-analysis covering the whole clinical trial program, and the upper bounds of the confidence intervals for MACE were < 1.3, fulfilling conventional definitions of cardiovascular safety. Tirzepatide was found to improve insulin sensitivity and insulin secretory responses to a greater extent than semaglutide, and this was associated with lower prandial insulin and glucagon concentrations. Both drugs caused similar reductions in appetite, although tirzepatide caused greater weight loss. While the clinical effects of tirzepatide have been very encouraging, important questions remain as to the mechanism of action. While GIP reduces food intake and body weight in rodents, these effects have not been demonstrated in humans. Moreover, it remains to be shown that GIPR agonism can improve insulin secretion in type 2 diabetic patients who have been noted in previous studies to be unresponsive to GIP. Certainly, the apparent advantage of tirzepatide, a dual incretin agonist, over GLP-1RA will spark renewed interest in the therapeutic potential of GIP in type 2 diabetes, obesity and related co-morbidities.
Keywords: Body weight; GIP/GLP-1 receptor co-agonists; GLP-1 receptor agonists; Glycemic control; HbA1c; Type 2 diabetes.
© 2022. The Author(s).
Conflict of interest statement
MAN has been member on advisory boards or has consulted with Boehringer Ingelheim, Eli Lilly & Co., Menarini/Berlin Chemie, Merck, Sharp & Dohme, NovoNordisk, Regor, and ShouTi Inc./Gasherbrum. He has received grant support from Merck, Sharp & Dohme. He is member of a data monitoring and safety board for Inventiva. He has also served on the speakers’ bureau of Eli Lilly & Co., Medscape, Medical Learning Institute, Menarini/Berlin Chemie, Merck, Sharp & Dohme, NovoNordisk, and Sun Pharmaceuticals. D.A.D. consults for Eli Lilly & Co. and Sun Pharmaceuticals.
Figures


References
-
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

