siRNA against CD40 delivered via a fungal recognition receptor ameliorates murine acute graft-versus-host disease
- PMID: 36051085
- PMCID: PMC9421973
- DOI: 10.1002/jha2.439
siRNA against CD40 delivered via a fungal recognition receptor ameliorates murine acute graft-versus-host disease
Abstract
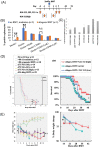
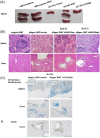
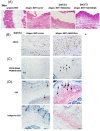
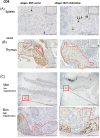
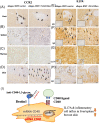
Acute graft-versus-host disease (aGvHD) remains a major threat to a successful outcome after allogeneic hematopoietic stem cell transplantation (HSCT). Although antibody-based targeting of the CD40/CD40 ligand costimulatory pathway can prevent aGvHD, side effects hampered their clinical application, prompting a need for other ways to interfere with this important dendritic T-cell costimulatory pathway. Here, we used small interfering RNA (siRNA) complexed with β-glucan allowing the binding and uptake of the siRNA/β-glucan complex (siCD40/schizophyllan [SPG]; chemical modifications called NJA-312, NJA-302, and NJA-515) into Dectin1+ cells, which recognize this pathogen-associated molecular pattern receptor. aGvHD was induced by the transplantation of splenocytes and bone marrow cells from C57BL/6J into CBF1 mice. Splenic dendritic cells retained Dectin1 expression after HSCT but showed lower expression after irradiation. The administration of siCD40/SPG, NJA-312, and NJA-302 ameliorated aGvHD-mediated lethality and tissue damage of spleen and liver, but not skin. Multiple NJA-312high injections prevented aGvHD but resulted in early weight loss in allogeneic HSCT mice. In addition, NJA-312 treatment caused delayed initial donor T and B-cell recovery but resulted in stable chimerism in surviving mice. Mechanistically, NJA-312 reduced organ damage by suppressing CCR2+, F4/80+, and IL17A-expressing cell accumulation in spleen, liver, and thymus but not the skin of mice with aGvHD. Our work demonstrates that siRNA targeting of CD40 delivered via the PAMP-recognizing lectin Dectin1 changes the immunological niche, suppresses organ-specific murine aGvHD, and induces immune tolerance after organ transplantation. Our work charts future directions for therapeutic interventions to modulate tissue-specific immune reactions using Pathogen-associated molecular pattern (PAMP) molecules like 1,3-β-glucan for cell delivery of siRNA.
Keywords: CCR2; CD40; Dectin1; IL17A; PAMP; acute GvHD; bone marrow transplantation; cytokine; siRNA.
© 2022 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

Similar articles
-
A novel β-glucan-oligonucleotide complex selectively delivers siRNA to APCs via Dectin-1.J Control Release. 2021 Oct 10;338:792-803. doi: 10.1016/j.jconrel.2021.09.011. Epub 2021 Sep 13. J Control Release. 2021. PMID: 34530053
-
Permanent acceptance of mouse cardiac allografts with CD40 siRNA to induce regulatory myeloid cells by use of a novel polysaccharide siRNA delivery system.Gene Ther. 2015 Mar;22(3):217-26. doi: 10.1038/gt.2014.119. Epub 2015 Jan 8. Gene Ther. 2015. PMID: 25567536
-
[Comparison of Acute Graft-Versus-Host Disease Mouse Models Established by TBI and BU/CY Conditioning Regimens].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022 Aug;30(4):1248-1254. doi: 10.19746/j.cnki.issn.1009-2137.2022.04.044. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022. PMID: 35981393 Chinese.
-
Icariin protects against acute graft-versus-host disease while preserving graft-versus-leukemia activity after allogeneic hematopoietic stem cell transplantation.Phytomedicine. 2024 Sep;132:155901. doi: 10.1016/j.phymed.2024.155901. Epub 2024 Jul 18. Phytomedicine. 2024. PMID: 39067193
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
-
The Role of the Plasminogen/Plasmin System in Inflammation of the Oral Cavity.Cells. 2023 Jan 30;12(3):445. doi: 10.3390/cells12030445. Cells. 2023. PMID: 36766787 Free PMC article. Review.
References
-
- Lu Y, Sakamaki S, Kuroda H, Kusakabe T, Konuma Y, Akiyama T, et al. Prevention of lethal acute graft‐versus‐host disease in mice by oral administration of T helper 1 inhibitor, TAK‐603. Blood. 2001;97:1123–30. - PubMed
-
- Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51. - PubMed
-
- Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–28. - PubMed
-
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
