Longitudinal changes in personalized platelet count metrics are good indicators of initial 3-year outcome in colorectal cancer
- PMID: 36051133
- PMCID: PMC9297428
- DOI: 10.12998/wjcc.v10.i20.6825
Longitudinal changes in personalized platelet count metrics are good indicators of initial 3-year outcome in colorectal cancer
Abstract
Background: Platelet count or complete blood count (CBC)-based ratios including lymphocyte-to-monocyte (LMR), neutrophil-to-lymphocyte (NLR), hemoglobin-to-platelet (HPR), red blood cell count distribution width-to-platelet (RPR), and platelet-to-lymphocyte (PLR) ratio are good predictors of colorectal cancer (CRC) survival. Their change in time is not well documented, however.
Aim: To investigate the effect of longitudinal CBC ratio changes on CRC survival and their possible associations with clinicopathological properties, comorbidities, and anamnestic data.
Methods: A retrospective longitudinal observational study was conducted with the inclusion of 835 CRC patients, who attended at Semmelweis University, Budapest. CBC ratios and two additional newly defined personalized platelet count metrics (pPLTD and pPLTS, the platelet counts relative to the measurement at the time of CRC diagnosis and to the one 4-6 wk after tumor removal surgery, respectively) were recorded.
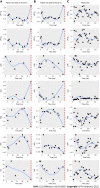
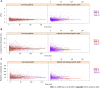
Results: The 835 CRC patients had a total of 4608 measurements (5.52 visits/patient, in average). Longitudinal survival models revealed that the increases/decreases in LMR [hazard ratio (HR): 0.4989, P < 0.0001], NLR (HR: 1.0819, P < 0.0001), HPR (HR: 0.0533, P = 0.0038), pPLTD (HR: 4.9229, P < 0.0001), and pPLTS (HR: 4.7568, P < 0.0001) values were poor prognostic signs of disease-specific survival. The same was obtained for all-cause mortality. Most abnormal changes occurred within the first 3 years after the diagnosis of CRC. RPR and PLR had an only marginal effect on disease-specific (P = 0.0675) and all-cause mortality (Bayesian 95% credible interval: 0.90-186.05), respectively.
Conclusion: LMR, NLR, and HPR are good metrics to follow the prognosis of the disease. pPLTD and pPLTS perform just as well as the former, while the use of RPR and PLR with the course of the disease is not recommended. Early detection of the abnormal changes in pPLTD, pPLTS, LMR, NLR, or HPR may alert the practicing oncologist for further therapy decisions in a timely manner.
Keywords: Colorectal neoplasms; Hemoglobin-to-platelet ratio; Lymphocyte-to-monocyte ratio; Neutrophil-to-lymphocyte ratio; Personalized platelet count; Platelet-to-lymphocyte ratio.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures






References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128–138. - PubMed
LinkOut - more resources
Full Text Sources

