Exploring discordant low amyloid beta and high neocortical tau positron emission tomography cases
- PMID: 36051174
- PMCID: PMC9413469
- DOI: 10.1002/dad2.12326
Exploring discordant low amyloid beta and high neocortical tau positron emission tomography cases
Abstract
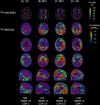
Introduction: Neocortical 3R4R (3-repeat/4-repeat) tau aggregates are rarely observed in the absence of amyloid beta (Aβ). 18F-MK6240 binds specifically to the 3R4R form of tau that is characteristic of Alzheimer's disease (AD). We report four cases with negative Aβ, but positive tau positron emission tomography (PET) findings.
Methods: All Australian Imaging, Biomarkers and Lifestyle study of aging (AIBL) study participants with Aβ (18F-NAV4694) and tau (18F-MK6240) PET scans were included. Centiloid <25 defined negative Aβ PET (Aβ-). The presence of neocortical tau was defined quantitatively and visually.
Results: Aβ- PET was observed in 276 participants. Four of these participants (one cognitively unimpaired [CU], two mild cognitive impairment [MCI], one AD) had tau tracer retention in a pattern consistent with Braak tau stages V to VI. Fluid biomarkers supported a diagnosis of AD. In silico analysis of APP, PSEN1, PSEN2, and MAPT genes did not identify relevant functional mutations.
Discussion: Discordant cases were infrequent (1.4% of all Aβ- participants). In these cases, the Aβ PET ligand may not be detecting the Aβ that is present.
Keywords: Alzheimer's disease; amyloid beta (Aβ); discordant; positron emission tomography; tau.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
This work was supported by the National Health and Medical Research Council (NHMRC) (grant numbers APP1140853, APP1132604). Natasha Krishnadas was supported by a cofunded PhD scholarship from Australian Rotary Health and the Estate of Bartolina Peluso. Simon M. Laws has received institutionally administered grants as follows: NHMRC APP1151854, APP1161706, APP1191535, APP2001320, APP2007656; Department of Health (Government of Western Australia) ‐ Multiple Sclerosis Western Australia ‐ Australian Alzheimer's Research Foundation ‐ Edith Cowan University, Strategic Research Centre Support ‐ Florey Institute of Neuroscience and Mental Health. Simon M. Laws has participated on the Cytox Group Ltd ‐ Scientific Advisory Board (pro bono). Victor L Villemagne has received consulting fees from IXICO, Eli Lilly, Life molecular imaging, and Hospicom, and has received payment/honoraria from ACE Barcelona. Christopher C. Rowe has received grants from Cerveau Technologies (institution), Eisai (institution), and Biogen (institution). Christopher C. Rowe has received consulting fees from Nutricia (speaker fee), Prothera, and Biogen (for preparation of educational material). Christopher C. Rowe has participated on a data safety board/advisory board for Cerveau Technologies (unpaid). Vincent Doré, Tenielle Porter, Fiona Lamb, and Svetlana Bozinovski do not report any disclosures.
Figures

References
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383‐388. - PubMed
-
- Masters CL, Beyreuther K. Alzheimer's centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain. 2006;129:2823‐2839. - PubMed
LinkOut - more resources
Full Text Sources
