Ferrets as a model for tuberculosis transmission
- PMID: 36051240
- PMCID: PMC9425069
- DOI: 10.3389/fcimb.2022.873416
Ferrets as a model for tuberculosis transmission
Abstract
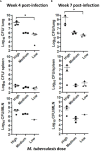
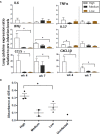
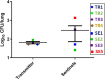
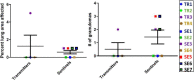
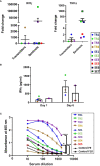
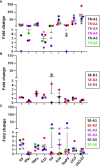
Even with the COVID-19 pandemic, tuberculosis remains a leading cause of human death due to a single infectious agent. Until successfully treated, infected individuals may continue to transmit Mycobacterium tuberculosis bacilli to contacts. As with other respiratory pathogens, such as SARS-CoV-2, modeling the process of person-to-person transmission will inform efforts to develop vaccines and therapies that specifically impede disease transmission. The ferret (Mustela furo), a relatively inexpensive, small animal has been successfully employed to model transmissibility, pathogenicity, and tropism of influenza and other respiratory disease agents. Ferrets can become naturally infected with Mycobacterium bovis and are closely related to badgers, well known in Great Britain and elsewhere as a natural transmission vehicle for bovine tuberculosis. Herein, we report results of a study demonstrating that within 7 weeks of intratracheal infection with a high dose (>5 x 103 CFU) of M. tuberculosis bacilli, ferrets develop clinical signs and pathological features similar to acute disease reported in larger animals, and ferrets infected with very-high doses (>5 x 104 CFU) develop severe signs within two to four weeks, with loss of body weight as high as 30%. Natural transmission of this pathogen was also examined. Acutely-infected ferrets transmitted M. tuberculosis bacilli to co-housed naïve sentinels; most of the sentinels tested positive for M. tuberculosis in nasal washes, while several developed variable disease symptomologies similar to those reported for humans exposed to an active tuberculosis patient in a closed setting. Transmission was more efficient when the transmitting animal had a well-established acute infection. The findings support further assessment of this model system for tuberculosis transmission including the testing of prevention measures and vaccine efficacy.
Keywords: animal model; ferret; mycobacterium; transmission; tuberculosis.
Copyright © 2022 Gupta, Somanna, Rowe, LaGatta, Helms, Owino, Jelesijevic, Harvey, Jacobs, Voss, Sakamoto, Day, Whalen, Karls, He, Tompkins, Bakre, Ross and Quinn.
Conflict of interest statement
Author NS was employed by GlaxoSmithKline and TV was employed by Merck Research Laboratories. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Beggs C. B., Noakes C. J., Sleigh P. A., Fletcher L. A., Siddiqi K. (2003). The transmission of tuberculosis in confined spaces: an analytical review of alternative epidemiological models. Int. J. Tuberc Lung Dis. 7 (11):1015–1026. - PubMed
-
- Bezos J., Álvarez-Carrión B., Rodríguez-Bertos A., Fernández-Manzano Á., de Juan L., Huguet C., et al. . (2016). Evidence of disseminated infection by mycobacterium avium subspecies hominissuis in a pet ferret (Mustela putorius furo). Res. Vet. Sci. 109:52–55. doi: 10.1016/j.rvsc.2016.09.013 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

