A comparison of three thromboprophylaxis regimens in critically ill COVID-19 patients: An analysis of real-world data
- PMID: 36051287
- PMCID: PMC9424612
- DOI: 10.3389/fcvm.2022.978420
A comparison of three thromboprophylaxis regimens in critically ill COVID-19 patients: An analysis of real-world data
Abstract
Introduction: Thrombotic complications of coronavirus disease 2019 (COVID-19) have received considerable attention. Although numerous conflicting findings have compared escalated thromboprophylaxis doses with a standard dose to prevent thrombosis, there is a paucity of literature comparing clinical outcomes in three different anticoagulation dosing regimens. Thus, we investigated the effectiveness and safety profiles of standard, intermediate, and high-anti-coagulation dosing strategies in COVID-19 critically ill patients.
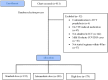
Methodology: This retrospective multicenter cohort study of intensive care unit (ICU) patients from the period of April 2020 to August 2021 in four Saudi Arabian centers. Inclusion criteria were age ≥ 18 years, diagnosis with severe or critical COVID-19 infection, and receiving prophylactic anticoagulant dose within 24-48 h of ICU admission. The primary endpoint was a composite of thrombotic events, with mortality rate and minor or major bleeding serving as secondary endpoints. We applied survival analyses with a matching weights procedure to control for confounding variables in the three arms.
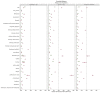
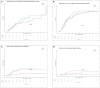
Results: A total of 811 patient records were reviewed, with 551 (standard-dose = 192, intermediate-dose = 180, and high-dose = 179) included in the analysis. After using weights matching, we found that the standard-dose group was not associated with an increase in the composite thrombotic events endpoint when compared to the intermediate-dose group {19.8 vs. 25%; adjusted hazard ratio (aHR) =1.46, [95% confidence of interval (CI), 0.94-2.26]} or when compared to high-dose group [19.8 vs. 24%; aHR = 1.22 (95% CI, 0.88-1.72)]. Also, there were no statistically significant differences in overall in-hospital mortality between the standard-dose and the intermediate-dose group [51 vs. 53.4%; aHR = 1.4 (95% CI, 0.88-2.33)] or standard-dose and high-dose group [51 vs. 61.1%; aHR = 1.3 (95% CI, 0.83-2.20)]. Moreover, the risk of major bleeding was comparable in all three groups [standard vs. intermediate: 4.8 vs. 2.8%; aHR = 0.8 (95% CI, 0.23-2.74); standard vs. high: 4.8 vs. 9%; aHR = 2.1 (95% CI, 0.79-5.80)]. However, intermediate-dose and high-dose were both associated with an increase in minor bleeding incidence with aHR = 2.9 (95% CI, 1.26-6.80) and aHR = 3.9 (95% CI, 1.73-8.76), respectively.
Conclusion: Among COVID-19 patients admitted to the ICU, the three dosing regimens did not significantly affect the composite of thrombotic events and mortality. Compared with the standard-dose regimen, intermediate and high-dosing thromboprophylaxis were associated with a higher risk of minor but not major bleeding. Thus, these data recommend a standard dose as the preferred regimen.
Keywords: COVID-19; critically ill patients; mortality; thromboprophylaxis; thromboprophylaxis doses.
Copyright © 2022 Alrashed, Cahusac, Mohzari, Bamogaddam, Alfaifi, Mathew, Alrumayyan, Alqahtani, Alshammari, AlNekhilan, Binrokan, Alamri, Alshahrani, Alshahrani, Alanazi, Alhassan, Alsaeed, Almutairi, Albujaidy, AlJuaid, Almalki, Ahmed, Alajami, Aljishi, Alsheef, Alajlan, Almutairi, Alsirhani, Alotaibi, Aljaber, Bahammam, Aldandan, Almulhim, Abraham and Alamer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

