Efficacy and safety of drug-coated balloons according to coronary vessel size. A report from the BASKET-SMALL 2 trial
- PMID: 36051841
- PMCID: PMC9421526
- DOI: 10.5114/aic.2022.118528
Efficacy and safety of drug-coated balloons according to coronary vessel size. A report from the BASKET-SMALL 2 trial
Abstract
Introduction: In BASKET-SMALL 2, drug-coated balloons (DCB) were non-inferior to drug-eluting stents (DES) in de-novo stenosis of small coronary vessels (≤ 2.75 mm) regarding clinical endpoints up to 36 months.
Aim: In the present subgroup analysis, we aimed to analyze the effect of the two treatment strategies in different vessel sizes.
Material and methods: Patients were analyzed according to the size of the device used (small > 2.5 mm vs. very small ≤ 2.5 mm). The primary endpoint was major adverse cardiac events (MACE), while secondary endpoints were target vessel revascularization (TVR), non-fatal myocardial infarction, cardiac death, and all-cause mortality, all at 36 months. Interactions for the different groups were assessed with Cox regression analysis.
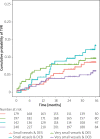
Results: Overall, 758 patients were enrolled in this analysis, of which 437 (58%) had very small vessel disease. There were similar results in both treatment groups for the primary endpoint in both small and very small vessels (DCB vs DES, MACE at 3 years in small vessels HR = 1.31, 95% CI: 0.74-2.32, p = 0.355, and very small vessels HR = 0.82, 95% CI: 0.49-1.39, p = 0.468). Second generation paclitaxel-eluting stents showed significantly higher rates for MACE (p = 0.041), TVR (p = 0.004) and non-fatal myocardial infarction (p = 0.036) compared to DCB in very small coronary arteries at 3 years, while results were similar in small coronary arteries.
Conclusions: Efficacy and safety of DCB are similar irrespective of vessel size. However, there is a beneficial effect of DCB over paclitaxel-eluting stents regarding TVR, non-fatal myocardial infarction and MACE that is most pronounced in very small coronary arteries.
Keywords: drug-coated balloon; drug-eluting stent; paclitaxel; small vessel disease; vessel size.
Copyright: © 2022 Termedia Sp. z o. o.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cutlip DE, Chauhan MS, Baim DS, et al. . Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol 2002; 40: 2082-9. - PubMed
-
- Kastrati A, Dibra A, Mehilli J, et al. . Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation 2006; 113: 2293/300. - PubMed
-
- George D. Dangas, Mehran R, et al. . In-stent restenosis in the drug-eluting stent era. J Am Coll Cadiol 2010; 56: 1897-907. - PubMed
-
- Kedhi E, Joesoef KS, McFadden E, et al. . Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 2010; 375: 201-9. - PubMed
-
- Harjai KJ, Kondareddy S, Pinkosky B, et al. . Everolimus-eluting stents versus sirolimus- or paclitaxel-eluting stents: two-year results from the Guthrie Health Off-Label Stent (GHOST) registry. J Interv Cardiol 2013; 26: 153-62. - PubMed
LinkOut - more resources
Full Text Sources
