EASL clinical practice guidelines: non-invasive liver tests for evaluation of liver disease severity and prognosis
- PMID: 36051951
- PMCID: PMC9380759
- DOI: 10.1136/flgastro-2021-102064
EASL clinical practice guidelines: non-invasive liver tests for evaluation of liver disease severity and prognosis
Abstract
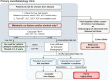
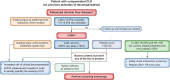
The European Association for the Study of the Liver has recently published updated guidelines on the use of non-invasive tests to identify and stratify chronic liver disease. Here, we provide a summary of the key recommendations from the guideline.
Keywords: alcoholic liver disease; hepatic fibrosis; liver function test; non-alcoholic steatohepatitis; screening.
© Author(s) (or their employer(s)) 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KWMA has received lecture honoraria from Intercept Pharma. AJA is an NIHR academic clinical fellow.
Figures
References
LinkOut - more resources
Full Text Sources
