Contribution of MMP14-expressing cancer-associated fibroblasts in the tumor immune microenvironment to progression of colorectal cancer
- PMID: 36052235
- PMCID: PMC9424903
- DOI: 10.3389/fonc.2022.956270
Contribution of MMP14-expressing cancer-associated fibroblasts in the tumor immune microenvironment to progression of colorectal cancer
Abstract
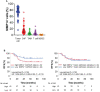
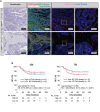
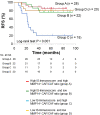
Matrix metalloproteinase 14 (MMP14) expression is implicated in progression of colorectal cancer, but its role in the tumor microenvironment (TME) has been unclear. The relevance of MMP14 to colorectal cancer progression was explored by analysis of transcriptomic data for colorectal adenocarcinoma patients (n = 592) in The Cancer Genome Atlas. The role of MMP14 in the TME was investigated in a retrospective analysis of tumor samples from 86 individuals with stage III colorectal cancer by single cell-based spatial profiling of MMP14 expression as performed by 12-color multiplex immunohistochemistry (mIHC). Analysis of gene expression data revealed that high MMP14 expression was associated with tumor progression and implicated both cancer-associated fibroblasts (CAFs) and tumor-associated macrophages in such progression. Spatial profiling by mIHC revealed that a higher percentage of MMP14+ cells among intratumoral CAFs (MMP14+ CAF/CAF ratio) was associated with poorer relapse-free survival. Multivariable analysis including key clinical factors identified the MMP14+ CAF/CAF ratio as an independent poor prognostic factor. Moreover, the patient subset with both a high MMP14+ CAF/CAF ratio and a low tumor-infiltrating lymphocyte density showed the worst prognosis. Our results suggest that MMP14+ CAFs play an important role in progression of stage III colorectal cancer and may therefore be a promising therapeutic target.
Keywords: M2 tumor-associated macrophages (M2-TAMs); cancer-associated fibroblast (CAF); colorectal cancer; matrix metalloproteinase 14 (MMP14); multiplex immunohistochemistry (mIHC).
Copyright © 2022 Makutani, Kawakami, Tsujikawa, Yoshimura, Chiba, Ito, Kawamura, Haratani and Nakagawa.
Conflict of interest statement
HK has received consulting fees from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., and Taiho Pharmaceutical Co. Ltd.YC honoraria from Bristol-Myers Squibb Co. Ltd., Bayer Yakuhin Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Merck Biopharma Co. Ltd., Takeda Pharmaceutical Co. Ltd., Yakult Pharmaceutical Industry, Teijin Pharma Ltd., and Taiho Pharmaceutical Co. Ltd.YC lecture fees from Glaxo Smith Kline K.K. and Otsuka Pharmaceutical Co. Ltd.YC and research funding from Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Kobayashi Pharmaceutical Co. Ltd., and Eisai Co. Ltd. TT has received speaker fees from MSD K.K., Ono Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. YC has received honoraria from Chugai Pharmaceutical Co. Ltd. KH has received lecture fees from AS ONE Corp., AstraZeneca K.K., Bristol-Myers Squibb Co. Ltd., MSD K.K., and Ono Pharmaceutical Co. Ltd. as well as research funding from AstraZeneca K.K. and MSD K.K. KN has received honoraria from Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Nanzando Co. Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Roche Diagnostics K.K., MSD K.K., Eli Lilly Japan K.K., Nippon Kayaku Co. Ltd., Daiichi Sankyo Co. Ltd., Novartis Pharma K.K., Kyowa Kirin Co. Ltd., Taiho Pharmaceutical Co. Ltd., Pfizer Japan Inc., AbbVie Inc., Bristol-Myers Squibb Co. Ltd., CareNet Inc., Amgen Inc., Medical Review Co. Ltd., Yodosha Co. Ltd., 3H Clinical Trial Inc., Thermo Fisher Scientific K.K., Hisamitsu Pharmaceutical Co. Inc., Nichi-Iko Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Medicus Shuppan Publishers Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Nikkei Business Publications Inc., Yomiuri Telecasting Corp., and Medical Mobile Communications Co. Ltd.YC research funding from MSD K.K., AstraZeneca K.K., Pfizer Japan Inc., ICON Japan K.K., Astellas Pharma Inc., Bayer Yakuhin Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., EPS International Co. Ltd., Bristol Myers Squibb Co. Ltd., CMIC Shift Zero K.K., PRA Health Sciences, Taiho Pharmaceutical Co. Ltd., Eisai Co. Ltd., Merck Biopharma Co. Ltd., Parexel International Corp., Mochida Pharmaceutical Co. Ltd., Covance Japan Inc., Ono Pharmaceutical Co. Ltd., Kissei Pharmaceutical Co. Ltd., Medical Research Support, Sysmex Corp., GlaxoSmithKline K.K., Sanofi K.K., A2 Healthcare Corp., Kyowa Hakko Kirin Co. Ltd., Syneos Health, AbbVie Inc., EPS Corp., Pfizer R&D Japan G.K., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., PPD-SNBL K.K., Nippon Boehringer Ingelheim Co. Ltd., IQVIA Services Japan K.K./Quintiles Inc., Japan Clinical Research Operations, and SymBio Pharmaceuticals Ltd.YC and consulting fees from Astellas Pharma Inc., Takeda Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc., Kyorin Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

