Outcomes of Partial Oral Antibiotic Treatment for Complicated Staphylococcus aureus Bacteremia in People Who Inject Drugs
- PMID: 36052413
- PMCID: PMC10169408
- DOI: 10.1093/cid/ciac714
Outcomes of Partial Oral Antibiotic Treatment for Complicated Staphylococcus aureus Bacteremia in People Who Inject Drugs
Abstract
Background: Staphylococcus aureus represents the leading cause of complicated bloodstream infections among persons who inject drugs (PWID). Standard of care (SOC) intravenous (IV) antibiotics result in high rates of treatment success but are not feasible for some PWID. Transition to oral antibiotics may represent an alternative treatment option.
Methods: We evaluated all adult patients with a history of injection drug use hospitalized from January 2016 through December 2021 with complicated S. aureus bloodstream infections, including infective endocarditis, epidural abscess, vertebral osteomyelitis, and septic arthritis. Patients were compared by antibiotic treatment (standard of care intravenous [SOC IV] antibiotics, incomplete IV therapy, or transition from initial IV to partial oral) using the primary composite endpoint of death or readmission from microbiologic failure within 90 days of discharge.
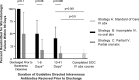
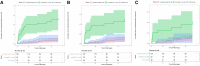
Results: Patients who received oral antibiotics after an incomplete IV antibiotic course were significantly less likely to experience microbiologic failure or death than patients discharged without oral antibiotics (P < .001). There was no significant difference in microbiologic failure rates when comparing patients who were discharged on partial oral antibiotics after receiving at least 10 days of IV antibiotics with SOC regimens (P > .9).
Conclusions: Discharge of PWID with partially treated complicated S. aureus bacteremias without oral antibiotics results in high rates of morbidity and should be avoided. For PWID hospitalized with complicated S. aureus bacteremias who have received at least 10 days of effective IV antibiotic therapy after clearance of bacteremia, transition to oral antibiotics with outpatient support represents a potential alternative if the patient does not desire SOC IV antibiotic therapy.
Keywords: Staphylococcus aureus; endocarditis; opioid use disorder; osteomyelitis; substance abuse.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. M. D. J. reports grants from CDC Epicenters (U54CK000609), NIH/NIDCR (K23DE029514), NIH/NIDA (R21DA053710), NIH/NIDCR (X01DE030402, X01DE030403, X01DE031119), NIH/NCATS (R21TR003410), and Pew Charitable Trusts; payment for lecture from American Hospital Association and Hektoen Institute for Medical Research (paid to author); payment for expert witness from Fisher Rushmer, Rouse Frets Write Gross, and Stanton Barton (paid to author); support for attending meetings and/or travel from Pew Charitable Trusts (Harnessing Health Systems to Expand and Enhance Antibiotic Stewardship in Outpatient Settings); and DSMB member for Duvelisib for treatment of COVID-19 (unpaid participation). A. A. reports paid work for University Children's Hospital Basel. J. M. reports the following grants or contracts unrelated to this work: PI, Swiss National Science Foundation, “Understanding the drivers of surgical site infections” (paid to institution in Switzerland); Sub-PI, CDC Prevention Epicenters Grant, “The Impact of An Existing Anesthesia Control Tower (ACT) Intervention to Improve Intraoperative Care on Infectious Outcomes” (paid to Washington University School of Medicine); Site PI (PI Philip Polgreen, University of Iowa), NCATS R21, “Determining the acceptability and feasibility of mobile-health approaches to gather clinical information from patients at home following hospital discharge (paid to Washington University School of Medicine). J. M. also reports consulting fees (payments <$10 000 per year to author) for work as a consultant on the topic of catheter-associated urinary tract infection surveillance for the Swiss National Center of Infection Control; role as Board Member for Swiss National Center for Infection Control (paid to institution in Switzerland) and role as Steering Committee Member for National Center for Antibiotic Resistance, Switzerland (unpaid); and other financial or non-financial interests as Co-PI (PI: Thomas Kessler, U of Zurich), Swiss National Science Foundation, Engineered Bacteriophages as Antibiotic Alternatives for Treating Catheter-Associated Urinary Tract Infections (CAUTIs) (unpaid). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Liu C, Bayer A, Cosgrove SE, et al. . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–55. - PubMed
-
- Baddour LM, Wilson WR, Bayer AS, et al. . Infective endocarditis in adults: diagnosis. Antimicrob Ther Manag Complic 2015;132:1435–86. - PubMed

