Galleria mellonella as an infection model for the virulent Mycobacterium tuberculosis H37Rv
- PMID: 36052440
- PMCID: PMC9481108
- DOI: 10.1080/21505594.2022.2119657
Galleria mellonella as an infection model for the virulent Mycobacterium tuberculosis H37Rv
Abstract
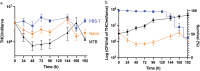
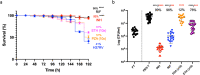
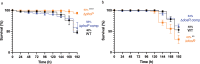
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a leading cause of infectious disease mortality. Animal infection models have contributed substantially to our understanding of TB, yet their biological and non-biological limitations are a research bottleneck. There is a need for more ethically acceptable, economical, and reproducible TB infection models capable of mimicking key aspects of disease. Here, we demonstrate and present a basic description of how Galleria mellonella (the greater wax moth, Gm) larvae can be used as a low cost, rapid, and ethically more acceptable model for TB research. This is the first study to infect Gm with the fully virulent MTB H37Rv, the most widely used strain in research. Infection of Gm with MTB resulted in a symptomatic lethal infection, the virulence of which differed from both attenuated Mycobacterium bovis BCG and auxotrophic MTB strains. The Gm-MTB model can also be used for anti-TB drug screening, although CFU enumeration from Gm is necessary for confirmation of mycobacterial load reducing activity of the tested compound. Furthermore, comparative virulence of MTB isogenic mutants can be determined in Gm. However, comparison of mutant phenotypes in Gm against conventional models must consider the limitations of innate immunity. Our findings indicate that Gm will be a practical, valuable, and advantageous additional model to be used alongside existing models to advance tuberculosis research.
Keywords: Galleria mellonella; Mycobacterium tuberculosis; Tuberculosis; infection model; innate immunity; mycobacteria.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
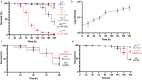
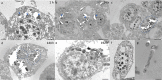
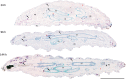
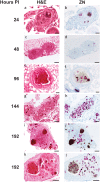

References
-
- Pagán AJ, Ramakrishnan L. The formation and function of granulomas. Annu Rev Immunol. 2018;36(1):639–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
