Fibroblast GSK-3α Promotes Fibrosis via RAF-MEK-ERK Pathway in the Injured Heart
- PMID: 36052698
- PMCID: PMC9481711
- DOI: 10.1161/CIRCRESAHA.122.321431
Fibroblast GSK-3α Promotes Fibrosis via RAF-MEK-ERK Pathway in the Injured Heart
Abstract
Background: Heart failure is the leading cause of mortality, morbidity, and health care expenditures worldwide. Numerous studies have implicated GSK-3 (glycogen synthase kinase-3) as a promising therapeutic target for cardiovascular diseases. GSK-3 isoforms seem to play overlapping, unique and even opposing functions in the heart. Previously, we have shown that of the 2 isoforms of GSK-3, cardiac fibroblast GSK-3β acts as a negative regulator of myocardial fibrosis in the ischemic heart. However, the role of cardiac fibroblast-GSK-3α in the pathogenesis of cardiac diseases is completely unknown.
Methods: To define the role of cardiac fibroblast-GSK-3α in myocardial fibrosis and heart failure, GSK-3α was deleted from fibroblasts or myofibroblasts with tamoxifen-inducible Tcf21- or Postn-promoter-driven Cre recombinase. Control and GSK-3α KO mice were subjected to cardiac injury and heart parameters were evaluated. The fibroblast kinome mapping was carried out to delineate molecular mechanism followed by in vivo and in vitro analysis.
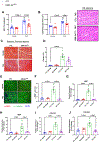
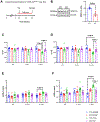
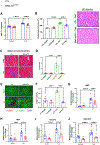
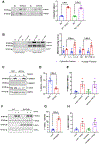
Results: Fibroblast-specific GSK-3α deletion restricted fibrotic remodeling and preserved function of the injured heart. We observed reductions in cell migration, collagen gel contraction, α-SMA protein levels, and expression of ECM genes in TGFβ1-treated KO fibroblasts, indicating that GSK-3α is required for myofibroblast transformation. Surprisingly, GSK-3α deletion did not affect SMAD3 activation, suggesting the profibrotic role of GSK-3α is SMAD3 independent. The molecular studies confirmed decreased ERK signaling in GSK-3α-KO CFs. Conversely, adenovirus-mediated expression of a constitutively active form of GSK-3α (Ad-GSK-3αS21A) in fibroblasts increased ERK activation and expression of fibrogenic proteins. Importantly, this effect was abolished by ERK inhibition.
Conclusions: GSK-3α-mediated MEK-ERK activation is a critical profibrotic signaling circuit in the injured heart, which operates independently of the canonical TGF-β1-SMAD3 pathway. Therefore, strategies to inhibit the GSK-3α-MEK-ERK signaling circuit could prevent adverse fibrosis in diseased hearts.
Keywords: cardiovascular diseases; fibroblasts; fibrosis; heart failure; myofibroblasts.
Conflict of interest statement
Disclosures
The authors have no conflicting interests to disclose concerning this work.
Figures




References
-
- Zannad F, Rossignol P and Iraqi W. Extracellular matrix fibrotic markers in heart failure. Heart Fail Rev. 2010;15:319–29. - PubMed
-
- Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D and Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008;14:467–74. - PubMed
-
- Zannad F, Alla F, Dousset B, Perez A and Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–6. - PubMed
-
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA and Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

