The N(2D) + CH2CHCN (Vinyl Cyanide) Reaction: A Combined Crossed Molecular Beam and Theoretical Study and Implications for the Atmosphere of Titan
- PMID: 36053010
- PMCID: PMC9483977
- DOI: 10.1021/acs.jpca.2c04263
The N(2D) + CH2CHCN (Vinyl Cyanide) Reaction: A Combined Crossed Molecular Beam and Theoretical Study and Implications for the Atmosphere of Titan
Abstract
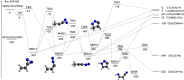
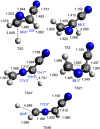
The reaction of electronically excited nitrogen atoms, N(2D), with vinyl cyanide, CH2CHCN, has been investigated under single-collision conditions by the crossed molecular beam (CMB) scattering method with mass spectrometric detection and time-of-flight (TOF) analysis at the collision energy, Ec, of 31.4 kJ/mol. Synergistic electronic structure calculations of the doublet potential energy surface (PES) have been performed to assist in the interpretation of the experimental results and characterize the overall reaction micromechanism. Statistical (Rice-Ramsperger-Kassel-Marcus, RRKM) calculations of product branching fractions (BFs) on the theoretical PES have been carried out at different values of temperature, including the one corresponding to the temperature (175 K) of Titan's stratosphere and at a total energy corresponding to the Ec of the CMB experiment. According to our theoretical calculations, the reaction is found to proceed via barrierless addition of N(2D) to the carbon-carbon double bond of CH2═CH-CN, followed by the formation of cyclic and linear intermediates that can undergo H, CN, and HCN elimination. In competition, the N(2D) addition to the CN group is also possible via a submerged barrier, leading ultimately to N2 + C3H3 formation, the most exothermic of all possible channels. Product angular and TOF distributions have been recorded for the H-displacement channels leading to the formation of a variety of possible C3H2N2 isomeric products. Experimentally, no evidence of CN, HCN, and N2 forming channels was observed. These findings were corroborated by the theory, which predicts a variety of competing product channels, following N(2D) addition to the double bond, with the main ones, at Ec = 31.4 kJ/mol, being six isomeric H forming channels: c-CH(N)CHCN + H (BF = 35.0%), c-CHNCHCN + H (BF = 28.1%), CH2NCCN + H (BF = 26.3%), c-CH2(N)CCN(cyano-azirine) + H (BF = 7.4%), trans-HNCCHCN + H (BF = 1.6%), and cis-HNCCHCN + H (BF = 1.3%), while C-C bond breaking channels leading to c-CH2(N)CH(2H-azirine) + CN and c-CH2(N)C + HCN are predicted to be negligible (0.02% and 0.2%, respectively). The highly exothermic N2 + CH2CCH (propargyl) channel is also predicted to be negligible because of the very high isomerization barrier from the initial addition intermediate to the precursor intermediate able to lead to products. The predicted product BFs are found to have, in general, a very weak energy dependence. The above cyclic and linear products containing an additional C-N bond could be potential precursors of more complex, N-rich organic molecules that contribute to the formation of the aerosols on Titan's upper atmosphere. Overall, the results are expected to have a significant impact on the gas-phase chemistry of Titan's atmosphere and should be properly included in the photochemical models.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Lindal G. F.; Wood G. E.; Hotz H. B.; Sweetnam D. N.; Eshleman V. R.; Tyler G. L. The Atmosphere of Titan: An Analysis of the Voyager 1 Radio Occultation Measurements. Icarus 1983, 53, 348–363. 10.1016/0019-1035(83)90155-0. - DOI
-
- Coustenis A.; Bezard B.; Gautier D. Titan’s Atmosphere from Voyager Infrared Observations: I. The Gas Composition of Titan’s Equatorial Rregion. Icarus 1989, 80, 54–76. 10.1016/0019-1035(89)90161-9. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

