Polymer-tethered glycosylated gold nanoparticles recruit sialylated glycoproteins into their protein corona, leading to off-target lectin binding
- PMID: 36053227
- PMCID: PMC9494357
- DOI: 10.1039/d2nr01818g
Polymer-tethered glycosylated gold nanoparticles recruit sialylated glycoproteins into their protein corona, leading to off-target lectin binding
Abstract
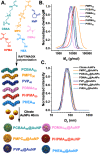
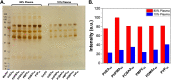
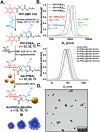
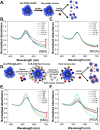
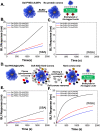
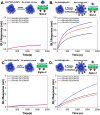
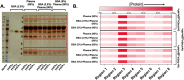
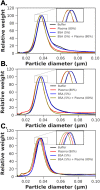
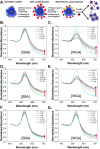
Upon exposure to biological fluids, the fouling of nanomaterial surfaces results in non-specific capture of proteins, which is particularly important when in contact with blood for in vivo and ex vivo applications. It is crucial to evaluate not just the protein components but also the glycans attached to those proteins. Polymer-tethered glycosylated gold nanoparticles have shown promise for use in biosensing/diagnostics, but the impact of the glycoprotein corona has not been established. Here we investigate how polymer-tethered glycosylated gold nanoparticles interact with serum proteins and demonstrate that the protein corona introduces new glycans and hence off-specific targeting capability. Using a panel of RAFT-derived polymers grafted to the gold surface, we show that the extent of corona formation is not dependent on the type of polymer. In lectin-binding assays, a glycan (galactose) installed on the chain-end of the polymer was available for binding even after protein corona formation. However, using sialic-acid binding lectins, it was found that there was significant off-target binding due to the large density of sialic acids introduced in the corona, confirmed by western blotting. To demonstrate the importance, we show that the nanoparticles can bind Siglec-2, an immune-relevant lectin post-corona formation. Pre-coating with (non-glycosylated) bovine serum albumin led to a significant reduction in the total glycoprotein corona. However, sufficient sialic acids were still present in the residual corona to lead to off-target binding. These results demonstrate the importance of the glycans when considering the protein corona and how 'retention of the desired function' does not rule out 'installation of undesired function' when considering the performance of glyco-nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Probing the glycans accessibility in the nanoparticle biomolecular corona.J Colloid Interface Sci. 2022 May;613:563-574. doi: 10.1016/j.jcis.2021.11.140. Epub 2021 Dec 16. J Colloid Interface Sci. 2022. PMID: 35066229
-
"Tuning aggregative versus non-aggregative lectin binding with glycosylated nanoparticles by the nature of the polymer ligand".J Mater Chem B. 2020 Jan 7;8(1):136-145. doi: 10.1039/c9tb02004g. Epub 2019 Nov 28. J Mater Chem B. 2020. PMID: 31778137
-
Surface roughness influences the protein corona formation of glycosylated nanoparticles and alter their cellular uptake.Nanoscale. 2019 Dec 28;11(48):23259-23267. doi: 10.1039/c9nr06835j. Epub 2019 Nov 29. Nanoscale. 2019. PMID: 31782458
-
High-affinity ligands of Siglec receptors and their therapeutic potentials.Curr Med Chem. 2011;18(23):3537-50. doi: 10.2174/092986711796642580. Curr Med Chem. 2011. PMID: 21756229 Review.
-
How Corona Formation Impacts Nanomaterials as Drug Carriers.Mol Pharm. 2020 Mar 2;17(3):725-737. doi: 10.1021/acs.molpharmaceut.9b01111. Epub 2020 Jan 24. Mol Pharm. 2020. PMID: 31939673 Review.
Cited by
-
Polymer-tethered glyconanoparticle colourimetric biosensors for lectin binding: structural and experimental parameters to ensure a robust output.RSC Adv. 2022 Nov 18;12(51):33080-33090. doi: 10.1039/d2ra06265h. eCollection 2022 Nov 15. RSC Adv. 2022. PMID: 36425181 Free PMC article.
-
Cell-mediated nanoparticle delivery systems: towards precision nanomedicine.Drug Deliv Transl Res. 2024 Nov;14(11):3032-3054. doi: 10.1007/s13346-024-01591-0. Epub 2024 Apr 13. Drug Deliv Transl Res. 2024. PMID: 38615157 Free PMC article. Review.
-
Effect of Gold Nanoparticles on the Conformation of Bovine Serum Albumin: Insights from CD Spectroscopic Analysis and Molecular Dynamics Simulations.ACS Omega. 2024 Dec 3;9(50):49283-49292. doi: 10.1021/acsomega.4c06409. eCollection 2024 Dec 17. ACS Omega. 2024. PMID: 39713703 Free PMC article.
-
Mass spectrometry-based top-down proteomics for proteoform profiling of protein coronas.Nat Protoc. 2025 Aug 5. doi: 10.1038/s41596-025-01229-6. Online ahead of print. Nat Protoc. 2025. PMID: 40764671 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

