The Dual Use of the Pyranine (HPTS) Fluorescent Probe: A Ground-State pH Indicator and an Excited-State Proton Transfer Probe
- PMID: 36053265
- PMCID: PMC9494743
- DOI: 10.1021/acs.accounts.2c00458
The Dual Use of the Pyranine (HPTS) Fluorescent Probe: A Ground-State pH Indicator and an Excited-State Proton Transfer Probe
Abstract
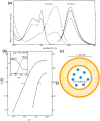
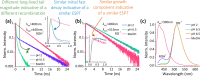
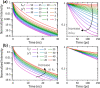
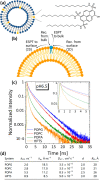
Molecular fluorescent probes are an essential experimental tool in many fields, ranging from biology to chemistry and materials science, to study the localization and other environmental properties surrounding the fluorescent probe. Thousands of different molecular fluorescent probes can be grouped into different families according to their photophysical properties. This Account focuses on a unique class of fluorescent probes that distinguishes itself from all other probes. This class is termed photoacids, which are molecules exhibiting a change in their acid-base transition between the ground and excited states, resulting in a large change in their pKa values between these two states, which is thermodynamically described using the Förster cycle. While there are many different photoacids, we focus only on pyranine, which is the most used photoacid, with pKa values of ∼7.4 and ∼0.4 for its ground and excited states, respectively. Such a difference between the pKa values is the basis for the dual use of the pyranine fluorescent probe. Furthermore, the protonated and deprotonated states of pyranine absorb and emit at different wavelengths, making it easy to focus on a specific state. Pyranine has been used for decades as a fluorescent pH indicator for physiological pH values, which is based on its acid-base equilibrium in the ground state. While the unique excited-state proton transfer (ESPT) properties of photoacids have been explored for more than a half-century, it is only recently that photoacids and especially pyranine have been used as fluorescent probes for the local environment of the probe, especially the hydration layer surrounding it and related proton diffusion properties. Such use of photoacids is based on their capability for ESPT from the photoacid to a nearby proton acceptor, which is usually, but not necessarily, water. In this Account, we detail the photophysical properties of pyranine, distinguishing between the processes in the ground state and the ones in the excited state. We further review the different utilization of pyranine for probing different properties of the environment. Our main perspective is on the emerging use of the ESPT process for deciphering the hydration layer around the probe and other parameters related to proton diffusion taking place while the molecule is in the excited state, focusing primarily on bio-related materials. Special attention is given to how to perform the experiments and, most importantly, how to interpret their results. We also briefly discuss the breadth of possibilities in making pyranine derivatives and the use of pyranine for controlling dynamic reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

