Urocortin 2 Gene Transfer for Systolic and Diastolic Dysfunction Due to Chronically Increased Left Ventricular Pressure
- PMID: 36053712
- PMCID: PMC9595638
- DOI: 10.1089/hum.2022.132
Urocortin 2 Gene Transfer for Systolic and Diastolic Dysfunction Due to Chronically Increased Left Ventricular Pressure
Abstract
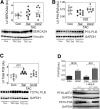
We used transverse aortic constriction (TAC) in mice to test the hypothesis that urocortin 2 (Ucn2) gene transfer would increase left ventricular (LV) systolic and diastolic function in the pressure-stressed LV. Three groups were studied: (1) control mice (no TAC); (2) mice that received saline 6 weeks after TAC; and (3) mice that received Ucn2 gene transfer 6 weeks after TAC, using adeno-associated virus 8 encoding murine Ucn2 (AAV8.mUcn2; 2 × 1013 genome copies (gc)/kg, i.v. per mouse). Echocardiography was performed 6 and 12 weeks after TAC. In terminal studies 12 weeks after TAC, rates of LV pressure development and decay and Tau were measured, and LV cardiac myocytes (CMs) were isolated and cytosolic Ca2+ transients and sarcomere shortening rates recorded. Reverse transcription polymerase chain reaction and immunoblotting were used to measure key proteins in LV samples. A CM cell line (HL-1) was used to explore mechanisms. Concentric LV hypertrophy was evident on echocardiography 6 weeks after TAC. Twelve weeks after TAC, LV ejection fraction (EF) was higher in mice that received Ucn2 gene transfer (TAC-saline: 65% ± 3%; TAC-Ucn2: 75% ± 2%; p = 0.01), as was LV peak +dP/dt (1.9-fold increase; p = 0.001) and LV peak -dP/dt (1.7-fold increase; p = 0.017). Tau was more rapid (23% reduction, p = 0.02), indicating improved diastolic function. The peak rates of sarcomere shortening (p = 0.002) and lengthening (p = 0.002) were higher in CMs from TAC-Ucn2 mice, and Tau was reduced (p = 0.001). LV (Ser-16) phosphorylation of phospholamban (PLB) was increased in TAC-Ucn2 mice (p = 0.025), and also was increased in HL-1 cells treated with angiotensin II to induce hypertrophy and incubated with Ucn2 peptide (p = 0.001). Ucn2 gene transfer in TAC-induced heart failure with preserved ejection fraction increased cardiac function in the intact LV and provided corresponding benefits in CMs isolated from study animals, including increased myofilament Ca2+ sensitivity during contraction. The mechanism includes enhanced CM Ca2+ handling associated with increased (Ser-16)-PLB.
Keywords: HL-1 cell line; TAC; Tau; gene transfer.
Conflict of interest statement
Dr. Hammond is a founder, board member, and unpaid consultant for Renova Therapeutics. Renova played no financial, intellectual, or editorial role in the studies. None of the other authors has disclosures.
Figures



Similar articles
-
Intravenous AAV8 Encoding Urocortin-2 Increases Function of the Failing Heart in Mice.Hum Gene Ther. 2015 Jun;26(6):347-56. doi: 10.1089/hum.2014.157. Epub 2015 Apr 9. Hum Gene Ther. 2015. PMID: 25760560 Free PMC article.
-
Urocortin 2 Gene Transfer Reduces the Adverse Effects of a Western Diet on Cardiac Function in Mice.Hum Gene Ther. 2019 Jun;30(6):693-701. doi: 10.1089/hum.2018.150. Epub 2019 Mar 11. Hum Gene Ther. 2019. PMID: 30648430 Free PMC article.
-
Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice.Hum Gene Ther. 2013 Sep;24(9):777-85. doi: 10.1089/hum.2013.088. Hum Gene Ther. 2013. PMID: 23931341 Free PMC article.
-
Urocortin 3 Gene Transfer Increases Function of the Failing Murine Heart.Hum Gene Ther. 2019 Jan;30(1):10-20. doi: 10.1089/hum.2018.103. Epub 2018 Oct 2. Hum Gene Ther. 2019. PMID: 30003813 Free PMC article.
-
Urocortin 2 Gene Transfer Improves Heart Function in Aged Mice.Mol Ther. 2020 Jan 8;28(1):180-188. doi: 10.1016/j.ymthe.2019.10.003. Epub 2019 Oct 29. Mol Ther. 2020. PMID: 31676153 Free PMC article.
References
-
- Barrick CJ, Rojas M, Schoonhoven R, et al. . Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: Temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 2007;292(5):H2119–H2130; doi: 10.1152/ajpheart.00816.2006 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

