The Interplay Between Autophagy and Regulated Necrosis
- PMID: 36053716
- PMCID: PMC10025850
- DOI: 10.1089/ars.2022.0110
The Interplay Between Autophagy and Regulated Necrosis
Abstract
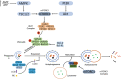
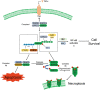
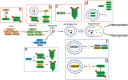
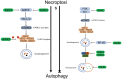
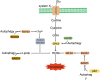
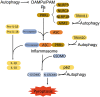
Significance: Autophagy is critical to cellular homeostasis. Emergence of the concept of regulated necrosis, such as necroptosis, ferroptosis, pyroptosis, and mitochondrial membrane-permeability transition (MPT)-derived necrosis, has revolutionized the research into necrosis. Both altered autophagy and regulated necrosis contribute to major human diseases. Recent studies reveal an intricate interplay between autophagy and regulated necrosis. Understanding the interplay at the molecular level will provide new insights into the pathophysiology of related diseases. Recent Advances: Among the three forms of autophagy, macroautophagy is better studied for its crosstalk with regulated necrosis. Macroautophagy seemingly can either antagonize or promote regulated necrosis, depending upon the form of regulated necrosis, the type of cells or stimuli, and other cellular contexts. This review will critically analyze recent advances in the molecular mechanisms governing the intricate dialogues between macroautophagy and main forms of regulated necrosis. Critical Issues: The dual roles of autophagy, either pro-survival or pro-death characteristics, intricate the mechanistic relationship between autophagy and regulated necrosis at molecular level in various pathological conditions. Meanwhile, key components of regulated necrosis are also involved in the regulation of autophagy, which further complicates the interrelationship. Future Directions: Resolving the controversies over causation between altered autophagy and a specific form of regulated necrosis requires approaches that are more definitive, where rigorous evaluation of autophagic flux and the development of more reliable and specific methods to quantify each form of necrosis will be essential. The relationship between chaperone-mediated autophagy or microautophagy and regulated necrosis remains largely unstudied. Antioxid. Redox Signal. 38, 550-580.
Keywords: autophagy; ferroptosis; mitochondrial membrane permeability transition; mitophagy; necroptosis; pyroptosis.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

Similar articles
-
Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation.Cells. 2021 Feb 28;10(3):515. doi: 10.3390/cells10030515. Cells. 2021. PMID: 33671004 Free PMC article. Review.
-
Partners in Crime: The Interplay of Proteins and Membranes in Regulated Necrosis.Int J Mol Sci. 2020 Mar 31;21(7):2412. doi: 10.3390/ijms21072412. Int J Mol Sci. 2020. PMID: 32244433 Free PMC article. Review.
-
Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies.Cells. 2020 Dec 17;9(12):2709. doi: 10.3390/cells9122709. Cells. 2020. PMID: 33348858 Free PMC article. Review.
-
Crosstalk between autophagy and other forms of programmed cell death.Eur J Pharmacol. 2025 May 15;995:177414. doi: 10.1016/j.ejphar.2025.177414. Epub 2025 Feb 20. Eur J Pharmacol. 2025. PMID: 39986593 Review.
-
Ferroptosis, necroptosis, and pyroptosis in the occurrence and development of ovarian cancer.Front Immunol. 2022 Jul 25;13:920059. doi: 10.3389/fimmu.2022.920059. eCollection 2022. Front Immunol. 2022. PMID: 35958626 Free PMC article. Review.
Cited by
-
The Effect of Natural Substances Contained in Bee Products on Prostate Cancer in In Vitro Studies.Molecules. 2023 Jul 28;28(15):5719. doi: 10.3390/molecules28155719. Molecules. 2023. PMID: 37570691 Free PMC article.
-
Cell death pathways: molecular mechanisms and therapeutic targets for cancer.MedComm (2020). 2024 Sep 4;5(9):e693. doi: 10.1002/mco2.693. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39239068 Free PMC article. Review.
-
ATP7B R778L mutant hepatocytes resist copper toxicity by activating autophagy and inhibiting necroptosis.Cell Death Discov. 2023 Sep 16;9(1):344. doi: 10.1038/s41420-023-01641-5. Cell Death Discov. 2023. PMID: 37717021 Free PMC article.
-
Drosophila model of HPV18-Induced pathogenesis reveals a role for E6 oncogene in regulation of NF-κB and Wnt to inhibit apoptosis.Tumour Virus Res. 2025 Jun;19:200316. doi: 10.1016/j.tvr.2025.200316. Epub 2025 Mar 10. Tumour Virus Res. 2025. PMID: 40074036 Free PMC article.
-
Mechanisms and Factors Influencing Resorption of Herniated Part of Lumbar Disc Herniation: Comprehensive Review.J Inflamm Res. 2025 Jun 30;18:8553-8562. doi: 10.2147/JIR.S525233. eCollection 2025. J Inflamm Res. 2025. PMID: 40620601 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

