Sexually dimorphic effects of SARM1 deletion on cardiac NAD+ metabolism and function
- PMID: 36053750
- PMCID: PMC9529255
- DOI: 10.1152/ajpheart.00370.2022
Sexually dimorphic effects of SARM1 deletion on cardiac NAD+ metabolism and function
Abstract
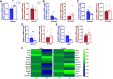
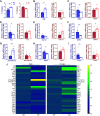
Nicotinamide adenine dinucleotide (NAD+) decline is repeatedly observed in heart disease and its risk factors. Although strategies promoting NAD+ synthesis to elevate NAD+ levels improve cardiac function, whether inhibition of NAD+ consumption can be therapeutic is less investigated. In this study, we examined the role of sterile-α and TIR motif containing 1 (SARM1) NAD+ hydrolase in mouse hearts, using global SARM1-knockout mice (KO). Cardiac function was assessed by echocardiography in male and female KO mice and wild-type (WT) controls. Hearts were collected for biochemical, histological, and molecular analyses. We found that the cardiac NAD+ pool was elevated in female KO mice, but only trended to increase in male KO mice. SARM1 deletion induced changes to a greater number of NAD+ metabolism transcripts in male mice than in female mice. Body weights, cardiac systolic and diastolic function, and geometry showed no changes in both male and female KO mice compared with WT counterparts. Male KO mice showed a small, but significant, elevation in cardiac collagen levels compared with WT counterparts, but no difference in collagen levels was detected in female mice. The increased collagen levels were associated with greater number of altered profibrotic and senescence-associated inflammatory genes in male KO mice, but not in female KO mice.NEW & NOTEWORTHY We examined the effects of SARM1 deletion on NAD+ pool, transcripts of NAD+ metabolism, and fibrotic pathway for the first time in mouse hearts. We observed the sexually dimorphic effects of SARM1 deletion. How these sex-dependent effects influence the outcomes of SARM1 deficiency in male and female mice in responses to cardiac stresses warrant further investigation. The elevation of cardiac NAD+ pool by SARM1 deletion provides evidence that targeting SARM1 may reverse disease-related NAD+ decline.
Keywords: NAD metabolism; SARM1; cardiac dysfunction; sex differences.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013. doi: 10.1016/j.cmet.2013.07.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

