Mechanical response of cardiac microtissues to acute localized injury
- PMID: 36053751
- PMCID: PMC9662801
- DOI: 10.1152/ajpheart.00305.2022
Mechanical response of cardiac microtissues to acute localized injury
Abstract
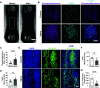
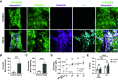
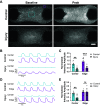
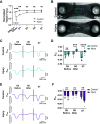
After a myocardial infarction (MI), the heart undergoes changes including local remodeling that can lead to regional abnormalities in mechanical and electrical properties, ultimately increasing the risk of arrhythmias and heart failure. Although these responses have been successfully recapitulated in animal models of MI, local changes in tissue and cell-level mechanics caused by MI remain difficult to study in vivo. Here, we developed an in vitro cardiac microtissue (CMT) injury system that through acute focal injury recapitulates aspects of the regional responses seen following an MI. With a pulsed laser, cell death was induced in the center of the microtissue causing a loss of calcium signaling and a complete loss of contractile function in the injured region and resulting in a 39% reduction in the CMT's overall force production. After 7 days, the injured area remained void of cardiomyocytes (CMs) and showed increased expression of vimentin and fibronectin, two markers for fibrotic remodeling. Interestingly, although the injured region showed minimal recovery, calcium amplitudes in uninjured regions returned to levels comparable with control. Furthermore, overall force production returned to preinjury levels despite the lack of contractile function in the injured region. Instead, uninjured regions exhibited elevated contractile function, compensating for the loss of function in the injured region, drawing parallels to changes in tissue-level mechanics seen in vivo. Overall, this work presents a new in vitro model to study cardiac tissue remodeling and electromechanical changes after injury.NEW & NOTEWORTHY We report an in vitro cardiac injury model that uses a high-powered laser to induce regional cell death and a focal fibrotic response within a human-engineered cardiac microtissue. The model captures the effects of acute injury on tissue response, remodeling, and electromechanical recovery in both the damaged region and surrounding healthy tissue, modeling similar changes to contractile function observed in vivo following myocardial infarction.
Keywords: cardiac fibrosis; cardiac mechanics; cardiac tissue engineering; iPSC-derived cardiomyocytes; organ-on-chip.
Conflict of interest statement
C.S.C. is a founder and own shares of Innolign Biomedical, a company that is developing engineered organ models for pharmaceutical research and development, and Satellite Biosciences, a company that is developing cell-based therapies. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

