Adding caplacizumab to standard of care in thrombotic thrombocytopenic purpura: a systematic review and meta-analysis
- PMID: 36053773
- PMCID: PMC10196763
- DOI: 10.1182/bloodadvances.2022008443
Adding caplacizumab to standard of care in thrombotic thrombocytopenic purpura: a systematic review and meta-analysis
Abstract
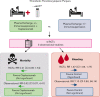
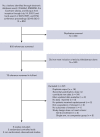
Immune thrombotic thrombocytopenic purpura (iTTP) is an acquired, fatal microangiopathy if untreated. Randomized controlled trials (RCTs) demonstrated faster time to response with addition of caplacizumab to standard of care (SOC). However, concerns about RCT selection bias and the high cost of caplacizumab warrant examination of all evidence, including real-world observational studies. In this systematic review and meta-analysis, we searched for comparative studies evaluating SOC with or without caplacizumab for the treatment of iTTP. We assessed risk of bias using the Cochrane risk-of-bias-2 tool (RCTs) and the Newcastle-Ottawa Scale (observational studies). The primary efficacy and safety outcomes were all-cause mortality and treatment-emergent bleeding, respectively. Secondary outcomes included exacerbation and relapse, refractory iTTP, and time to response. We included 2 high-quality RCTs and 3 observational studies at high risk of bias comprising 632 total participants. Compared with SOC, caplacizumab was associated with a nonsignificant reduction in the relative risk [RR] of death in RCTs (RR, 0.21; 95% confidence interval [CI], 0.05-1.74) and observational studies (RR, 0.62; 95% CI, 0.07-4.41). Compared with SOC, caplacizumab was associated with an increased bleeding risk in RCTs (RR, 1.37; 95% CI, 1.06-1.77). In observational studies, bleeding risk was not significantly increased (RR, 7.10; 95% CI, 0.90-56.14). Addition of caplacizumab was associated with a significant reduction in refractory iTTP and exacerbation risks and shortened response time but increased relapse risk. Frontline addition of caplacizumab does not significantly reduce all-cause mortality compared with SOC alone, although it reduces refractory disease risk, shortens time to response, and improves exacerbation rates at the expense of increased relapse and bleeding risk.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.C. has served as a consultant for Synergy and has received authorship royalties from UpToDate. A.M.P. has received grant funding on behalf of her institution from Sanofi Genzyme. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Caplacizumab as frontline therapy in addition to standard treatment in iTTP.Blood Adv. 2023 May 23;7(10):2129-2131. doi: 10.1182/bloodadvances.2022009448. Blood Adv. 2023. PMID: 36469553 Free PMC article. No abstract available.
Similar articles
-
Efficacy and safety of caplacizumab in the treatment of thrombotic thrombocytopenic purpura: a systematic review and meta-analysis.Expert Rev Hematol. 2023 May;16(5):377-385. doi: 10.1080/17474086.2023.2202850. Epub 2023 Apr 14. Expert Rev Hematol. 2023. PMID: 37045600
-
Management of immune thrombotic thrombocytopenic purpura with caplacizumab: a Canadian, single-centre, real-world experience.Platelets. 2023 Dec;34(1):2157807. doi: 10.1080/09537104.2022.2157807. Platelets. 2023. PMID: 36636834
-
Should we consider caplacizumab as routine treatment for acute thrombotic thrombocytopenic purpura? An expert perspective on the pros and cons.Expert Rev Hematol. 2024 Jan-Mar;17(1-3):9-25. doi: 10.1080/17474086.2024.2318347. Epub 2024 Feb 20. Expert Rev Hematol. 2024. PMID: 38353182 Review.
-
Real-world effectiveness of caplacizumab vs the standard of care in immune thrombotic thrombocytopenic purpura.Blood Adv. 2022 Dec 27;6(24):6219-6227. doi: 10.1182/bloodadvances.2022008028. Blood Adv. 2022. PMID: 35930694 Free PMC article.
-
Impact of first-line use of caplacizumab on treatment outcomes in immune thrombotic thrombocytopenic purpura.J Thromb Haemost. 2023 Mar;21(3):559-572. doi: 10.1016/j.jtha.2022.11.010. Epub 2022 Dec 22. J Thromb Haemost. 2023. PMID: 36696206
Cited by
-
Global Health Resource Utilization and Cost-Effectiveness of Therapeutics and Diagnostics in Immune Thrombotic Thrombocytopenic Purpura (TTP).J Clin Med. 2023 Jul 25;12(15):4887. doi: 10.3390/jcm12154887. J Clin Med. 2023. PMID: 37568288 Free PMC article. Review.
-
Iatrogenic hemorrhage and extensive venous thromboembolism during iTTP treatment with caplacizumab-A case report.EJHaem. 2024 Jun 10;5(4):768-771. doi: 10.1002/jha2.949. eCollection 2024 Aug. EJHaem. 2024. PMID: 39157617 Free PMC article.
-
A Long-Term Follow-Up Study in Immune-Mediated Thrombotic Thrombocytopenic Purpura: What Are the Outcomes?J Clin Med. 2023 Nov 25;12(23):7305. doi: 10.3390/jcm12237305. J Clin Med. 2023. PMID: 38068357 Free PMC article.
-
Current state and potential applications of neonatal Fc receptor (FcRn) inhibitors in hematologic conditions.Am J Hematol. 2024 Dec;99(12):2351-2366. doi: 10.1002/ajh.27487. Epub 2024 Sep 26. Am J Hematol. 2024. PMID: 39324647 Review.
-
More on the use of frontline caplacizumab in immune-mediated thrombotic thrombocytopenic purpura.Blood Adv. 2023 Jun 27;7(12):2678-2680. doi: 10.1182/bloodadvances.2022009021. Blood Adv. 2023. PMID: 36260763 Free PMC article. No abstract available.
References
-
- Cuker A, Cataland SR, Coppo P, et al. Redefining outcomes in immune TTP: an international working group consensus report. Blood. 2021;137(14):1855–1861. - PubMed

