Markers of neutrophil mediated inflammation associate with disturbed continuous electroencephalogram after out of hospital cardiac arrest
- PMID: 36053856
- PMCID: PMC10087484
- DOI: 10.1111/aas.14145
Markers of neutrophil mediated inflammation associate with disturbed continuous electroencephalogram after out of hospital cardiac arrest
Abstract
Background: Achieving an acceptable neurological outcome in cardiac arrest survivors remains challenging. Ischemia-reperfusion injury induces inflammation, which may cause secondary neurological damage. We studied the association of ICU admission levels of inflammatory biomarkers with disturbed 48-hour continuous electroencephalogram (cEEG), and the association of the daily levels of these markers up to 72 h with poor 6-month neurological outcome.
Methods: This is an observational, post hoc sub-study of the COMACARE trial. We measured serum concentrations of procalcitonin (PCT), high-sensitivity C-reactive protein (hsCRP), osteopontin (OPN), myeloperoxidase (MPO), resistin, and proprotein convertase subtilisin/kexin type 9 (PCSK9) in 112 unconscious, mechanically ventilated ICU-treated adult OHCA survivors with initial shockable rhythm. We used grading of 48-hour cEEG monitoring as a measure for the severity of the early neurological disturbance. We defined 6-month cerebral performance category (CPC) 1-2 as good and CPC 3-5 as poor long-term neurological outcome. We compared the prognostic value of biomarkers for 6-month neurological outcome to neurofilament light (NFL) measured at 48 h.
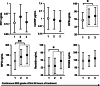
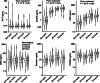
Results: Higher OPN (p = .03), MPO (p < .01), and resistin (p = .01) concentrations at ICU admission were associated with poor grade 48-hour cEEG. Higher levels of ICU admission OPN (OR 3.18; 95% CI 1.25-8.11 per ln[ng/ml]) and MPO (OR 2.34; 95% CI 1.30-4.21) were independently associated with poor 48-hour cEEG in a multivariable logistic regression model. Poor 6-month neurological outcome was more common in the poor cEEG group (63% vs. 19% p < .001, respectively). We found a significant fixed effect of poor 6-month neurological outcome on concentrations of PCT (F = 7.7, p < .01), hsCRP (F = 4.0, p < .05), and OPN (F = 5.6, p < .05) measured daily from ICU admission to 72 h. However, the biomarkers did not have independent predictive value for poor 6-month outcome in a multivariable logistic regression model with 48-hour NFL.
Conclusion: Elevated ICU admission levels of OPN and MPO predicted disturbances in cEEG during the subsequent 48 h after cardiac arrest. Thus, they may provide early information about the risk of secondary neurological damage. However, the studied inflammatory markers had little value for long-term prognostication compared to 48-hour NFL.
Keywords: cardiac arrest; continuous electroencephalogram; hypoxia; inflammation; ischemia; neutrophilic granulocyte; postcardiac arrest syndrome; prognostication; reperfusion injury; seizures.
© 2022 The Authors. Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
Giuseppe Ristagno has served at data monitoring committee for DSMB trial: TRansfusion Strategies in Acute Brain INjured Patients (TRAIN).
Kaj Blennow has received consulting fees from Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Lilly, MagQu, Prothena, Roche Diagnostics and Siemens Healthineers; honoraria for lectures from: GEECD/Roche Diagnostics and IFCC/SNIBE; has served at data monitoring committees for: Julius Clinical and Novartis and he is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Henrik Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Jussi Toppila has received consulting/lecture fees from: UCB Pharma Finland and SGS Fimko Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

