Efficacy and safety of SARS-CoV-2 vaccination in patients with inflammatory bowel disease on immunosuppressive and biological therapy: Prospective observational study
- PMID: 36054100
- PMCID: PMC9439210
- DOI: 10.1371/journal.pone.0273612
Efficacy and safety of SARS-CoV-2 vaccination in patients with inflammatory bowel disease on immunosuppressive and biological therapy: Prospective observational study
Abstract
Background and aims: SARS-CoV-2 is a worldwide serious health problem and vaccination seems to have a crucial role in managing the COVID-19 pandemic. The aim of this prospective observational study was to monitor the trend of antibodies against SARS-CoV-2 after vaccination with BNT162b2 (COMIRNATY) in patients with inflammatory bowel disease treated by immunosuppressive and/or biological therapy, demonstrate whether any type of this therapy is associated with poorer production of antibodies against COVID-19 and evaluate the safety of vaccination against COVID-19 in these patients.
Methods: Eighty-seven eligible patients from one tertiary gastroenterological center with inflammatory bowel disease (60 with CD, 27 with UC) treated by immunosuppressive and/or biological therapy from the antiTNFα group were indicated to vaccination against SARS-CoV-2. Effectiveness of vaccination was evaluated by the values of antibodies before and 4 weeks after 2nd dose of vaccine. Additional goal was to evaluate adverse events of vaccination.
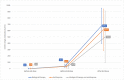
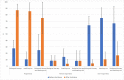
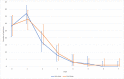
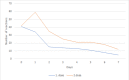
Results: Before the 2nd dose of vaccine, geometric mean of SARS-CoV-2 IgG antibodies were 40.7 U/ml in the biological therapy group, 34.8 U/ml in the azathioprine group and 44.8 U/ml in the combination therapy group of patients. The geometric means were 676.5.7 U/ml in the biological therapy group, 614.4 U/ml in the azathioprine group and 500.1 U/ml in the combination therapy group of patients four weeks after 2nd dose. Statistically significant differences between these groups were not proved. Several non-severe local and general adverse events were present in our patients with a majority of these events on the day of vaccine administration and the day after, no anaphylactic reactions were present.
Conclusions: Our measurements proved the efficacy and safety of vaccination against SARS-CoV-2 in patients with inflammatory bowel disease treated by immunosuppressive and/or biological therapy. Statistically significant differences between our groups of patients were not proved.
Conflict of interest statement
he authors have declared that no competing interests exist.
Figures
Similar articles
-
Anti-SARS-CoV-2 Vaccination and Antibody Response in Patients With Inflammatory Bowel Disease on Immune-modifying Therapy: Prospective Single-Tertiary Study.Inflamm Bowel Dis. 2022 Oct 3;28(10):1506-1512. doi: 10.1093/ibd/izab301. Inflamm Bowel Dis. 2022. PMID: 34849919
-
Antibody Response to SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease: Results of a Single-Center Cohort Study in a Tertiary Hospital in Germany.Dig Dis. 2022;40(6):719-727. doi: 10.1159/000521343. Epub 2021 Dec 10. Dig Dis. 2022. PMID: 34915480 Free PMC article.
-
Effect of anti-SARS-CoV-2 BNT162b2 mRNA vaccination on thrombin generation in children with inflammatory bowel disease.Front Immunol. 2023 Oct 30;14:1257072. doi: 10.3389/fimmu.2023.1257072. eCollection 2023. Front Immunol. 2023. PMID: 37965328 Free PMC article.
-
MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature.J Neurol. 2022 Oct;269(10):5198-5212. doi: 10.1007/s00415-022-11194-9. Epub 2022 Jun 23. J Neurol. 2022. PMID: 35737110 Free PMC article. Review.
-
Effectiveness and safety of SARS-CoV-2 vaccine in Inflammatory Bowel Disease patients: a systematic review, meta-analysis and meta-regression.Aliment Pharmacol Ther. 2022 May;55(10):1244-1264. doi: 10.1111/apt.16913. Epub 2022 Mar 30. Aliment Pharmacol Ther. 2022. PMID: 35355306 Free PMC article.
Cited by
-
Critical illness in immunocompromised patients: insights into relapse or persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): case series report.J Med Case Rep. 2025 Jul 27;19(1):371. doi: 10.1186/s13256-025-05431-8. J Med Case Rep. 2025. PMID: 40717099 Free PMC article.
-
Efficacy of COVID-19 vaccines in inflammatory bowel disease patients receiving anti-TNF therapy: A systematic review and meta-analysis.Heliyon. 2023 Sep 5;9(9):e19609. doi: 10.1016/j.heliyon.2023.e19609. eCollection 2023 Sep. Heliyon. 2023. PMID: 37810049 Free PMC article.
-
Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland.J Clin Med. 2024 Oct 17;13(20):6173. doi: 10.3390/jcm13206173. J Clin Med. 2024. PMID: 39458123 Free PMC article.
References
-
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al.. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020. May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 - DOI - PMC - PubMed
-
- Feldman M, Friedman L, Bramdt L, et al.. Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology, diagnosis, management. 11th ed. Philadelphia (PA): Elsevier; 2021. p. 1868–1929.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

