Role of FruR transcriptional regulator in virulence of Listeria monocytogenes and identification of its regulon
- PMID: 36054213
- PMCID: PMC9439231
- DOI: 10.1371/journal.pone.0274005
Role of FruR transcriptional regulator in virulence of Listeria monocytogenes and identification of its regulon
Abstract
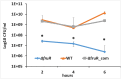
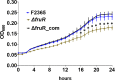
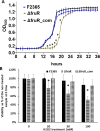
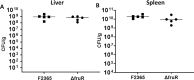
Listeria monocytogenes is a ubiquitous opportunistic foodborne pathogen capable of survival in various adverse environmental conditions. Pathogenesis of L. monocytogenes is tightly controlled by a complex regulatory network of transcriptional regulators that are necessary for survival and adaptations to harsh environmental conditions both inside and outside host cells. Among these regulatory pathways are members of the DeoR-family transcriptional regulators that are known to play a regulatory role in sugar metabolism. In this study, we deciphered the role of FruR, a DeoR family protein, which is a fructose operon transcriptional repressor protein, in L. monocytogenes pathogenesis and growth. Following intravenous (IV) inoculation in mice, a mutant strain with deletion of fruR exhibited a significant reduction in bacterial burden in liver and spleen tissues compared to the parent strain. Further, the ΔfruR strain had a defect in cell-to-cell spread in L2 fibroblast monolayers. Constitutive activation of PrfA, a pleiotropic activator of L. monocytogenes virulence factors, did not restore virulence to the ΔfruR strain, suggesting that the attenuation was not a result of impaired PrfA activation. Transcriptome analysis revealed that FruR functions as a positive regulator for genes encoding enzymes involved in the pentose phosphate pathway (PPP) and as a repressor for genes encoding enzymes in the glycolysis pathway. These results suggested that FruR may function to facilitate NADPH regeneration, which is necessary for full protection from oxidative stress. Interestingly, deletion of fruR increased sensitivity of L. monocytogenes to H2O2, confirming a role for FruR in survival of L. monocytogenes during oxidative stress. Using anti-mouse neutrophil/monocyte monoclonal antibody RB6-8C5 (RB6) in an in vivo infection model, we found that FruR has a specific function in protecting L. monocytogenes from neutrophil/monocyte-mediated killing. Overall, this work clarifies the role of FruR in controlling L. monocytogenes carbon flow between glycolysis and PPP for NADPH homeostasis, which provides a new mechanism allowing metabolic adaptation of L. monocytogenes to oxidative stress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Freitag NE (2006) From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression. Future Microbiology 1: 89–101. - PubMed
-
- Centers for Disease C, Prevention (1998) Multistate outbreak of listeriosis—United States, 1998. MMWR Morb Mortal Wkly Rep 47: 1085–1086. - PubMed
-
- Centers for Disease C, Prevention (2011) Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe—United States, August-September 2011. MMWR Morb Mortal Wkly Rep 60: 1357–1358. - PubMed
-
- Centers for Disease C, Prevention (2000) Multistate outbreak of listeriosis—United States, 2000. MMWR Morb Mortal Wkly Rep 49: 1129–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

