Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19
- PMID: 36054369
- PMCID: PMC9538270
- DOI: 10.1002/lary.30353
Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19
Abstract
Background and aims: Olfactory dysfunction is a recognized manifestation in patients infected with Coronavirus Disease 2019 (COVID-19). This investigation aimed to assess the effect of mometasone furoate intranasal spray on the improvement of smell dysfunction in post-COVID-19 patients.
Materials and methods: This randomized placebo-controlled trial included 80 non-hospitalized adult patients who had persistent anosmia or severe microsmia for more than 4 weeks due to COVID-19 infection. The participants were randomly allocated to the intervention or placebo group to receive mometasone furoate nasal spray or sodium chloride intranasal spray during 4 weeks of follow-up, respectively. The patients' olfactory dysfunction was assessed in terms of visual analog scale (VAS), and smell test score according to the modified version of the University of Pennsylvania smell identification test for the Iranian population.
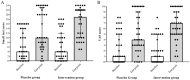
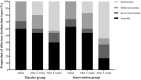
Results: A total of 70 participants completed the follow-up period and were analyzed in this study. By comparing the olfactory scores including smell test and VAS scores, no significant differences were found between case and control groups at baseline, 2, and 4 weeks intervals. However, the change of both olfactory scores at pre to post-treatment intervals and 2-4 weeks was significantly higher in the mometasone group relative to the placebo group. At post-treatment, the frequency of anosmia was 22.9% reduced in the case group compared to the control group.
Conclusion: Overall, there was no significant difference in olfactory dysfunction between the two groups during follow-up. However, based on the significant between-group difference in terms of olfactory scores changes, it seems that the nasal corticosteroids may be a positive effect on the recovery process of patients who received more than 2 weeks.
Level of evidence: 2 Laryngoscope, 132:2209-2216, 2022.
Keywords: COVID-19; UPSIT; VAS score; intranasal corticosteroid; olfactory dysfunction.
© 2022 The American Laryngological, Rhinological and Otological Society, Inc.
Figures
Comment in
-
In response to Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19.Laryngoscope. 2023 Apr;133(4):E31. doi: 10.1002/lary.30506. Epub 2022 Dec 10. Laryngoscope. 2023. PMID: 36495295 Free PMC article. No abstract available.
-
In reference to Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19.Laryngoscope. 2023 Apr;133(4):E29-E30. doi: 10.1002/lary.30505. Epub 2022 Dec 10. Laryngoscope. 2023. PMID: 36495301 Free PMC article.
Similar articles
-
Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: A randomized, double blind clinical trial.Int Immunopharmacol. 2021 Sep;98:107871. doi: 10.1016/j.intimp.2021.107871. Epub 2021 Jun 12. Int Immunopharmacol. 2021. PMID: 34147912 Free PMC article. Clinical Trial.
-
Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial.Am J Otolaryngol. 2021 Mar-Apr;42(2):102884. doi: 10.1016/j.amjoto.2020.102884. Epub 2021 Jan 4. Am J Otolaryngol. 2021. PMID: 33429174 Free PMC article. Clinical Trial.
-
Interventions for the prevention of persistent post-COVID-19 olfactory dysfunction.Cochrane Database Syst Rev. 2021 Jul 22;7(7):CD013877. doi: 10.1002/14651858.CD013877.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Sep 5;9:CD013877. doi: 10.1002/14651858.CD013877.pub3. PMID: 34291812 Free PMC article. Updated.
-
Alpha-lipoic acid does not improve olfactory training results in olfactory loss due to COVID-19: a double-blind randomized trial.Braz J Otorhinolaryngol. 2024 Jan-Feb;90(1):101356. doi: 10.1016/j.bjorl.2023.101356. Epub 2023 Oct 30. Braz J Otorhinolaryngol. 2024. PMID: 37944311 Free PMC article. Clinical Trial.
-
Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies.PLoS One. 2023 Aug 2;18(8):e0288285. doi: 10.1371/journal.pone.0288285. eCollection 2023. PLoS One. 2023. PMID: 37531338 Free PMC article.
Cited by
-
Updated Clinical Practice Guidelines for the Diagnosis and Management of Long COVID.Infect Chemother. 2024 Mar;56(1):122-157. doi: 10.3947/ic.2024.0024. Epub 2024 Mar 13. Infect Chemother. 2024. PMID: 38527781 Free PMC article. Review.
-
Magnetic resonance spectroscopy findings of brain olfactory areas in patients with COVID-19-related anosmia: A preliminary comparative study.World J Otorhinolaryngol Head Neck Surg. 2023 Aug 28;10(2):105-112. doi: 10.1002/wjo2.132. eCollection 2024 Jun. World J Otorhinolaryngol Head Neck Surg. 2023. PMID: 38855283 Free PMC article.
-
The Effect of Corticosteroids on Post-Covid-19 Smell Loss: A Meta-Analysis.Iran J Otorhinolaryngol. 2023 Sep;35(130):235-246. doi: 10.22038/IJORL.2023.72451.3456. Iran J Otorhinolaryngol. 2023. PMID: 38090618 Free PMC article.
-
Nasal sprays for treating COVID-19: a scientific note.Pharmacol Rep. 2023 Apr;75(2):249-265. doi: 10.1007/s43440-023-00463-7. Epub 2023 Feb 27. Pharmacol Rep. 2023. PMID: 36848033 Free PMC article. Review.
-
Efficacy of Gabapentin For Post-COVID-19 Olfactory Dysfunction: The GRACE Randomized Clinical Trial.JAMA Otolaryngol Head Neck Surg. 2023 Dec 1;149(12):1111-1119. doi: 10.1001/jamaoto.2023.2958. JAMA Otolaryngol Head Neck Surg. 2023. PMID: 37733356 Free PMC article. Clinical Trial.
References
-
- Zhang J‐j, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. - PubMed
-
- WHO . Coronavirus disease 2019 (COVID‐19): situation report, 191. 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

