Characterization of pathogen-inactivated COVID-19 convalescent plasma and responses in transfused patients
- PMID: 36054476
- PMCID: PMC9538076
- DOI: 10.1111/trf.17083
Characterization of pathogen-inactivated COVID-19 convalescent plasma and responses in transfused patients
Abstract
Background: Efficacy of donated COVID-19 convalescent plasma (dCCP) is uncertain and may depend on antibody titers, neutralizing capacity, timing of administration, and patient characteristics.
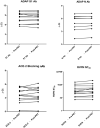
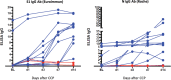
Study design and methods: In a single-center hypothesis-generating prospective case-control study with 1:2 matched dCCP recipients to controls according to disease severity at day 1, hospitalized adults with COVID-19 pneumonia received 2 × 200 ml pathogen-reduced treated dCCP from 2 different donors. We evaluated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in COVID-19 convalescent plasma donors and recipients using multiple antibody assays including a Coronavirus antigen microarray (COVAM), and binding and neutralizing antibody assays. Outcomes were dCCP characteristics, antibody responses, 28-day mortality, and dCCP -related adverse events in recipients.
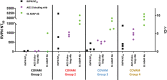
Results: Eleven of 13 dCCPs (85%) contained neutralizing antibodies (nAb). PRT did not affect dCCP antibody activity. Fifteen CCP recipients and 30 controls (median age 64 and 65 years, respectively) were enrolled. dCCP recipients received 2 dCCPs from 2 different donors after a median of one hospital day and 11 days after symptom onset. One dCCP recipient (6.7%) and 6 controls (20%) died (p = 0.233). We observed no dCCP-related adverse events. Transfusion of unselected dCCP led to heterogeneous SARS CoV-2 antibody responses. COVAM clustered dCCPs in 4 distinct groups and showed endogenous immune responses to SARS-CoV-2 antigens over 14-21 days post dCCP in all except 4 immunosuppressed recipients.
Discussion: PRT did not impact dCCP anti-virus neutralizing activity. Transfusion of unselected dCCP did not impact survival and had no adverse effects. Variable dCCP antibodies and post-transfusion antibody responses indicate the need for controlled trials using well-characterized dCCP with informative assays.
Keywords: COVID-19; COVID-19 convalescent plasma; SARS-CoV2; neutralizing antibodies; pathogen-reduction treatment.
© 2022 The Authors. Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
LI collaborates with Cerus. Anil Bagri, Johannes Irsch, and Laurence Corash are employees of Cerus. Maja Weisser, Karoline Leuzinger, Hans Pargger, Nikolaus Deigendesch, Anil Bagri, and Nina Khanna have no conflict of interest. Michael Paul Busch, Graham Simmons, and Mars Stone are employees of Vitalant Research and have no conflict of interest. Philip L. Felgner, Rafael R de Assis, and Saahir Khan are employees of the University of California and the University of Southern California and have no conflict of interest. Cheng‐ting Tsai, Peter V Robinson, and David Seftel are employees of Enable Biosciences—manufacturers of the ADAP technology assays which are not yet approved for commercial use.
Figures



References
-
- Center JHCR. Johns Hopkins coronavirus resource Center 2021‐06‐04, 2021. Accessed 2021‐06‐04.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

