In vitro cytocompatibility and antibacterial studies on biodegradable Zn alloys supplemented by a critical assessment of direct contact cytotoxicity assay
- PMID: 36054531
- PMCID: PMC10086991
- DOI: 10.1002/jbm.b.35147
In vitro cytocompatibility and antibacterial studies on biodegradable Zn alloys supplemented by a critical assessment of direct contact cytotoxicity assay
Abstract
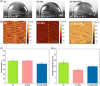
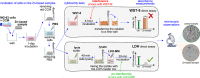
In vitro cytotoxicity assessment is indispensable in developing new biodegradable implant materials. Zn, which demonstrates an ideal corrosion rate between Mg- and Fe-based alloys, has been reported to have excellent in vivo biocompatibility. Therefore, modifications aimed at improving Zn's mechanical properties should not degrade its biological response. As sufficient strength, ductility and corrosion behavior required of load-bearing implants has been obtained in plastically deformed Zn-3Ag-0.5Mg, the effect of simultaneous Ag and Mg additions on in vitro cytocompatibility and antibacterial properties was studied, in relation to Zn and Zn-3Ag. Direct cell culture on samples and indirect extract-based tests showed almost no significant differences between the tested Zn-based materials. The diluted extracts of Zn, Zn-3Ag, and Zn-3Ag-0.5Mg showed no cytotoxicity toward MG-63 cells at a concentration of ≤12.5%. The cytotoxic effect was observed only at high Zn2+ ion concentrations and when in direct contact with metallic samples. The highest LD50 (lethal dose killing 50% of cells) of 13.4 mg/L of Zn2+ ions were determined for the Zn-3Ag-0.5Mg. Similar antibacterial activity against Escherichia coli and Staphylococcus aureus was observed for Zn and Zn alloys, so the effect is attributed mainly to the released Zn2+ ions exhibiting bactericidal properties. Most importantly, our experiments indicated the limitations of water-soluble tetrazolium salt-based cytotoxicity assays for direct tests on Zn-based materials. The discrepancies between the WST-8 assay and SEM observations are attributed to the interference of Zn2+ ions with tetrazolium salt, therefore favoring its transformation into formazan, giving false cell viability quantitative results.
Keywords: Zn alloys; antibacterial properties; biodegradation; cytotoxicity; tetrazolium salt-based assay.
© 2022 The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

