Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: A randomised clinical trial
- PMID: 36054556
- PMCID: PMC10086806
- DOI: 10.1111/ceo.14148
Low-concentration atropine eyedrops for myopia control in a multi-racial cohort of Australian children: A randomised clinical trial
Abstract
Background: To test the hypothesis that 0.01% atropine eyedrops are a safe and effective myopia-control approach in Australian children.
Methods: Children (6-16 years; 49% Europeans, 18% East Asian, 22% South Asian, and 12% other/mixed ancestry) with documented myopia progression were enrolled into this single-centre randomised, parallel, double-masked, placebo-controlled trial and randomised to receive 0.01% atropine (n = 104) or placebo (n = 49) eyedrops (2:1 ratio) instilled nightly over 24 months (mean index age = 12.2 ± 2.5 and 11.2 ± 2.8 years, respectively). Outcome measures were the changes in spherical equivalent (SE) and axial length (AL) from baseline.
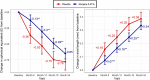
Results: At 12 months, the mean SE and AL change from baseline were -0.31D (95% confidence interval [CI] = -0.39 to -0.22) and 0.16 mm (95%CI = 0.13-0.20) in the atropine group and -0.53D (95%CI = -0.66 to -0.40) and 0.25 mm (95%CI = 0.20-0.30) in the placebo group (group difference p ≤ 0.01). At 24 months, the mean SE and AL change from baseline was -0.64D (95%CI = -0.73 to -0.56) and 0.34 mm (95%CI = 0.30-0.37) in the atropine group, and -0.78D (95%CI = -0.91 to -0.65) and 0.38 mm (95%CI = 0.33-0.43) in the placebo group. Group difference at 24 months was not statistically significant (p = 0.10). At 24 months, the atropine group had reduced accommodative amplitude and pupillary light response compared to the placebo group.
Conclusions: In Australian children, 0.01% atropine eyedrops were safe, well-tolerated, and had a modest myopia-control effect, although there was an apparent decrease in efficacy between 18 and 24 months, which is likely driven by a higher dropout rate in the placebo group.
Keywords: atropine; axial length; myopia; myopia control; randomised controlled trial.
© 2022 The Authors. Clinical & Experimental Ophthalmology published by John Wiley & Sons Australia, Ltd on behalf of Royal Australian and New Zealand College of Ophthalmologists.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology. 2012;119(2):347‐354. - PubMed
-
- Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(2):451‐457 e451. - PubMed
-
- Chia A, Lu QS, Tan D. Five‐year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% Eyedrops. Ophthalmology. 2016;123(2):391‐399. - PubMed
-
- Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285‐2291. - PubMed
-
- Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572‐579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources