The Genetic Landscape of Complex Childhood-Onset Hyperkinetic Movement Disorders
- PMID: 36054588
- PMCID: PMC9804670
- DOI: 10.1002/mds.29182
The Genetic Landscape of Complex Childhood-Onset Hyperkinetic Movement Disorders
Abstract
Background and objective: The objective of this study was to better delineate the genetic landscape and key clinical characteristics of complex, early-onset, monogenic hyperkinetic movement disorders.
Methods: Patients were recruited from 14 international centers. Participating clinicians completed standardized proformas capturing demographic, clinical, and genetic data. Two pediatric movement disorder experts reviewed available video footage, classifying hyperkinetic movements according to published criteria.
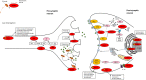
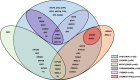
Results: One hundred forty patients with pathogenic variants in 17 different genes (ADCY5, ATP1A3, DDC, DHPR, FOXG1, GCH1, GNAO1, KMT2B, MICU1, NKX2.1, PDE10A, PTPS, SGCE, SLC2A1, SLC6A3, SPR, and TH) were identified. In the majority, hyperkinetic movements were generalized (77%), with most patients (69%) manifesting combined motor semiologies. Parkinsonism-dystonia was characteristic of primary neurotransmitter disorders (DDC, DHPR, PTPS, SLC6A3, SPR, TH); chorea predominated in ADCY5-, ATP1A3-, FOXG1-, NKX2.1-, SLC2A1-, GNAO1-, and PDE10A-related disorders; and stereotypies were a prominent feature in FOXG1- and GNAO1-related disease. Those with generalized hyperkinetic movements had an earlier disease onset than those with focal/segmental distribution (2.5 ± 0.3 vs. 4.7 ± 0.7 years; P = 0.007). Patients with developmental delay also presented with hyperkinetic movements earlier than those with normal neurodevelopment (1.5 ± 2.9 vs. 4.7 ± 3.8 years; P < 0.001). Effective disease-specific therapies included dopaminergic agents for neurotransmitters disorders, ketogenic diet for glucose transporter deficiency, and deep brain stimulation for SGCE-, KMT2B-, and GNAO1-related hyperkinesia.
Conclusions: This study highlights the complex phenotypes observed in children with genetic hyperkinetic movement disorders that can lead to diagnostic difficulty. We provide a comprehensive analysis of motor semiology to guide physicians in the genetic investigation of these patients, to facilitate early diagnosis, precision medicine treatments, and genetic counseling. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: chorea; dystonia; hyperkinetic movement disorders; infantile parkinsonism; myoclonus.
© 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures

References
-
- Kurian MA, Gissen P, Smith M, et al. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol 2011;10:721–733. - PubMed
-
- Dale RC, Grattan‐Smith P, Nicholson M, et al. Microdeletions detected using chromosome microarray in children with suspected genetic movement disorders: a single‐centre study. Dev Med Child Neurol 2012;54:618–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

