Clinical and Molecular Determinants of Clonal Evolution in Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria
- PMID: 36054881
- PMCID: PMC10476808
- DOI: 10.1200/JCO.22.00710
Clinical and Molecular Determinants of Clonal Evolution in Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria
Abstract
Purpose: Secondary myeloid neoplasms (sMNs) remain the most serious long-term complications in patients with aplastic anemia (AA) and paroxysmal nocturnal hemoglobinuria (PNH). However, sMNs lack specific predictors, dedicated surveillance measures, and early therapeutic interventions.
Patients and methods: We studied a multicenter, retrospective cohort of 1,008 patients (median follow-up 8.6 years) with AA and PNH to assess clinical and molecular determinants of clonal evolution.
Results: Although none of the patients transplanted upfront (n = 117) developed clonal complications (either sMN or secondary PNH), the 10-year cumulative incidence of sMN in nontransplanted cases was 11.6%. In severe AA, older age at presentation and lack of response to immunosuppressive therapy were independently associated with increased risk of sMN, whereas untreated patients had the highest risk among nonsevere cases. The elapsed time from AA to sMN was 4.5 years. sMN developed in 94 patients. The 5-year overall survival reached 40% and was independently associated with bone marrow blasts at sMN onset. Myelodysplastic syndrome with high-risk phenotypes, del7/7q, and ASXL1, SETBP1, RUNX1, and RAS pathway gene mutations were the most frequent characteristics. Cross-sectional studies of clonal dynamics from baseline to evolution revealed that PIGA/human leukocyte antigen lesions decreased over time, being replaced by clones with myeloid hits. PIGA and BCOR/L1 mutation carriers had a lower risk of sMN progression, whereas myeloid driver lesions marked the group with a higher risk.
Conclusion: The risk of sMN in AA is associated with disease severity, lack of response to treatment, and patients' age. sMNs display high-risk morphological, karyotypic, and molecular features. The landscape of acquired somatic mutations is complex and incompletely understood and should be considered with caution in medical management.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
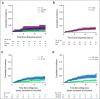

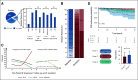
References
-
- Bacigalupo A: How I treat acquired aplastic anemia. Blood 129:1428-1436, 2017 - PubMed
-
- Peffault de Latour R, Kulasekararaj A, Iacobelli S, et al. : Eltrombopag added to immunosuppression in severe aplastic anemia. N Engl J Med 386:11-23, 2022 - PubMed
-
- Dameshek W: Riddle: What do aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH) and “hypoplastic” leukemia have in common? Blood 30:251-254, 1967 - PubMed

