Epo-IGF1R cross talk expands stress-specific progenitors in regenerative erythropoiesis and myeloproliferative neoplasm
- PMID: 36054916
- PMCID: PMC9837451
- DOI: 10.1182/blood.2022016741
Epo-IGF1R cross talk expands stress-specific progenitors in regenerative erythropoiesis and myeloproliferative neoplasm
Abstract
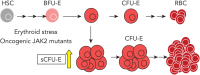
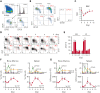
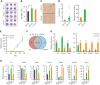
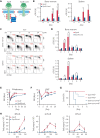
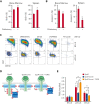
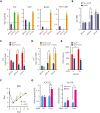
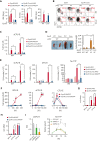
We found that in regenerative erythropoiesis, the erythroid progenitor landscape is reshaped, and a previously undescribed progenitor population with colony-forming unit-erythroid (CFU-E) activity (stress CFU-E [sCFU-E]) is expanded markedly to restore the erythron. sCFU-E cells are targets of erythropoietin (Epo), and sCFU-E expansion requires signaling from the Epo receptor (EpoR) cytoplasmic tyrosines. Molecularly, Epo promotes sCFU-E expansion via JAK2- and STAT5-dependent expression of IRS2, thus engaging the progrowth signaling from the IGF1 receptor (IGF1R). Inhibition of IGF1R and IRS2 signaling impairs sCFU-E cell growth, whereas exogenous IRS2 expression rescues cell growth in sCFU-E expressing truncated EpoR-lacking cytoplasmic tyrosines. This sCFU-E pathway is the major pathway involved in erythrocytosis driven by the oncogenic JAK2 mutant JAK2(V617F) in myeloproliferative neoplasm. Inability to expand sCFU-E cells by truncated EpoR protects against JAK2(V617F)-driven erythrocytosis. In samples from patients with myeloproliferative neoplasm, the number of sCFU-E-like cells increases, and inhibition of IGR1R and IRS2 signaling blocks Epo-hypersensitive erythroid cell colony formation. In summary, we identified a new stress-specific erythroid progenitor cell population that links regenerative erythropoiesis to pathogenic erythrocytosis.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.K.R. has received consulting fees from Constellation, Incyte, Celgene/BMS, Novartis, Promedior, CTI, Jazz Pharmaceuticals, Blueprint, Stemline, Galecto, Pharmaessentia, Abbvie, Sierra Oncology, and Disc Medicines. The remaining authors declare no competing financial interests.
Figures

References
-
- Constantinescu SN, Ghaffari S, Lodish HF. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends Endocrinol Metab. 1999;10(1):18–23. - PubMed
-
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83(1):59–67. - PubMed
-
- Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84(3):331–334. - PubMed
-
- Darnell JE., Jr. STATs and gene regulation. Science. 1997;277(5332):1630–1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

